What is radioactivity?
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Radioactive Compounds and Their Clinical Application
1. The atomic structure, 2. Radioactive processes, 3. Radioactive decay, 4. Penetration potential, 5. Quantification of radioactivity
What
is radioactivity?
The atomic structure
It is fundamental to look at the structure of
an atom in order to understand what radioactivity is. As discussed in Chapter 1
and briefly summarised here, an atom consists of a positively charged nucleus
formed from the so-called nucleons, which is surrounded by negatively charged
electrons, which may occupy different energy levels. Protons and neutrons form
the nucleus. Protons are nucleons with a positive charge and a mass of 1.6726 ×
10−24 g. The atomic number (Z)
expresses the number of protons. A neutron is a nucleon without a charge and a
mass similar to that of protons (1.6749 × 10−24 g). The so-called
neutron number (N) describes the
total number of neutrons. Neutrons and protons are held together by nuclear
binding forces and therefore form the nucleus. The letter A stands for the number of nucleons, which is the sum of number of
protons (Z) and neutrons (N). Electrons have a mass of 9.1094 × 10−28
g and they move in energy levels around the nucleus. Lower orbitals, which are
defined as the orbitals closer to the nucleus, possess a higher kinetic energy.
If an electron moves to an orbital closer to the nucleus, energy is released,
whilst the energy is required to move it
The number of electrons
should be equal to the number of protons in order to have an element without
any charge. Typically, the number of neutrons equals the number of protons
(Figure 10.1).
Radioactive processes
Radioactive decay, also known as radioactivity, describes the process by
which an unstable nucleus spon-taneously loses energy in order to form a stable
nucleus. This energy loss is achieved by emitting particles of ionising
radiation. An element or material that spontaneously emits energy in this form
is considered as radioactive. Radiation can take place in the form of α, β−,
β+, X-rays and γ-rays.
Radioactivity is defined as the process whereby an unstable
nucleus spontaneously loses energy in order
to form a more stable nucleus.
The terms nuclide
and radionuclide describe
identifiable atomic species (nonradioactive or radioactive), which are
characterised by their exact number of protons (Z) and neutrons (N). In
contrast, an element is only defined by its number of protons (Z). The number of neutrons can vary,
which leads to different isotopes of the same element as described in Chapter
1. An element can have a number of isotopes, some of which are stable and some
are not stable and therefore are classified as radioactive.
Radioactive decay
It is important to understand the different forms of radioactive
decay, as not all forms of radiation are useful for clinical applications. It
is also crucial to understand the reason for the occurrence of radioactivity in
order to provide the patient with the best and safest possible treatment
option.
There are two reasons for the occurrence of radioactive decay:
one is that the ratio of neutrons to protons is greater or less than 1. The
second reason is that radioactive decay takes places as a result of an energy
imbalance within the atom, which means that the atom needs to get rid of energy
in order to reach a stable form. The different forms of radiation can be
summarised as α, β, γ-decay and X-ray emission.
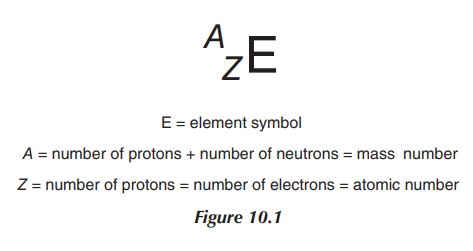
Alpha-(α-)radiation is defined as the emission of
helium particles, precisely 42He2+. α-Decay occurs in elements with a so-called heavy nucleus, principally in elements
with a higher atomic mass (typically in ele-ments with Z > 82).
α-Particles are fairly heavy particles and follow a straight path when
penetrating through a material. They only display a short-range activity and
the radiation can be easily shielded off with a piece
Their clinical application is very limited and
includes only a few therapeutic examples. Current research includes the use of
monoclonal antibodies as radiopharmaceuticals. The idea is to deliver
radioiso-topes directly to the tumour cells, minimising the exposure of healthy
cells to radiation.
In general, interaction of α-radiation with neighbouring matter
can occur in two ways – through ionisation or excitation. Excitation means that
an α-particle can, upon collision, promote an electron to a higher energy level
(higher outer shell). Once the electron falls back to its original energy
level, energy is emitted. The more important interaction is the ionisation of
an atom. This occurs when an α-particle collides with its target and ‘strips’
away an electron, leaving behind a positively charged molecule (Figure 10.2).
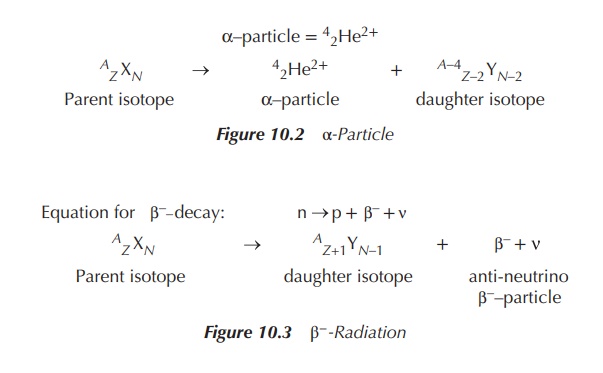
Beta-(β-)decay occurs when basically an electron
is ejected from the nucleus. This occurs when the ‘neutron to proton ratio’ is >1. In
order for this to happen, within the nucleus a neutron is converted into a
proton and a negatively charged β-particle (negatron, β−).
Additionally a so-called antineutrino ( ) is ‘produced’, carrying away any
excess binding energy from the nucleus. These processes result in an increase
in the proton number (Z) to Z + 1 and a decrease in the neutron
number (N) to N − 1. Negatively charged β-particles have the appearance of
electrons, but they originate from the nucleus and carry energy. In contrast,
electrons that are present in the orbit outside the nucleus have no energy and
obviously their origin differs (Figure 10.3).
β-Particles differ significantly from α-particles as they are
considered as extremely light and fast particles. As a consequence, they travel
much further and their clinical applications include imaging methods as well as
therapeutic ones, with the emphasis being on the latter.
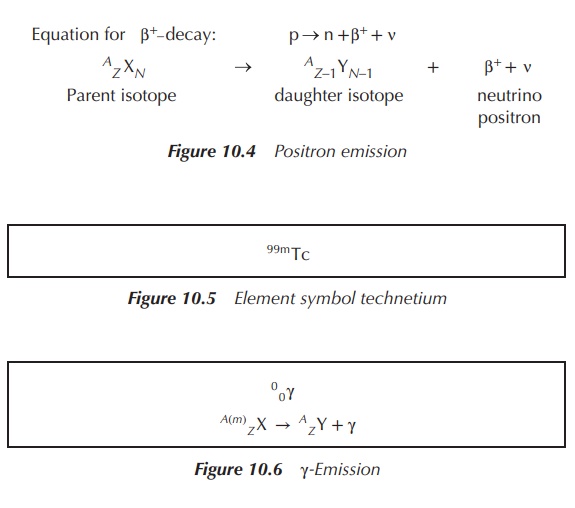
Positron
emission is the ejection of positively charged β-particles from a proton-rich nucleus. This occurs when the neutron to proton ratio is <1. For this to happen, a proton is converted
into a neutron, a positron (β+) and a neutrino (v). A neutrino is the opposite of an antineutrino, a small particle
carrying no mass or charge. These processes result in a decrease in the proton
number (Z) to Z − 1 and an increase in the neutron number (N) to N + 1. The
differences between a positron and a neutron manifest in a lower energy and
range for positrons. Positrons and positron-emitting elements are used mainly
for imaging purposes in the so-called positron emission tomography (PET, see
Chapter 10) (Figure 10.4).
Gamma-(γ-)emission is the elimination of excess energy by the emission of photons. A γ-photon has no charge or mass and occurs as electromagnetic radiation. The radiation occurs at short wavelengths and is therefore of high energy. It has the longest range of all nuclear emissions discussed. The nucleus that emits the γ-photon does not undergo any change of the neutron number; mostly isomers are formed. Isomers are defined as nuclides of the same atomic mass (A) and number (Z) with the only difference being that one isomer is in an excited (metastable (m)) state. This is marked with AmE as the atomic number (Figures 10.5 and 10.6).
γ-Emission normally occurs following another nuclear decay. It
has a high penetrating power of several metres as the γ-photons are not
charged. They typically interact with matter through direct collision with
nuclei and electrons of the orbital. γ-Emission finds its clinical application
mainly as part of radiopharmaceutical imaging processes.
Electron
capture and X-ray emission constitutes another form of positron emission
and occurs in unstable atoms where
the ‘neutron to proton’ ratio is <1. In
order to convert to a stable atom, a proton from the nucleus catches an
electron from the orbital and transforms into a neutron and a neutrino, with
the neutrino carrying any excess energy. Typically, a cascade reaction follows,
in which electrons from the outer (higher energy) orbitals move closer to the
nucleus and fill the vacated orbitals. Orbitals closer to the nucleus are of
lower energy, and the energy difference is given off as X-rays, a form of
electromagnetic radiation. In contrast to γ-decay, which originates
predominantly from the nucleus, X-rays stem from outside the nucleus.
Additionally, X-rays have a wavelength longer than γ-photons. The result of
electron capture and X-rays are the so-called isobars, similar to β-decay.
Isobars are nuclides with the same mass number (A) but different atomic
number (Z) and different neutron number
(N).
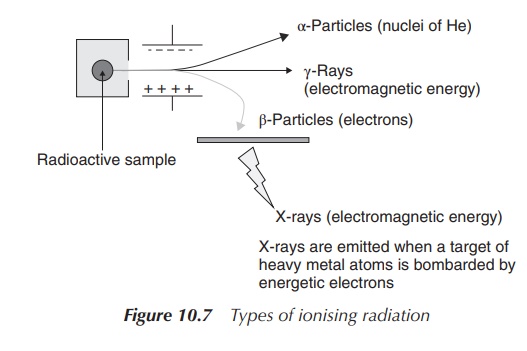
Figure 10.7 shows the effect of charge on
different forms of radiation. A radioactive sample is placed in a container,
where radiation is released in only one direction. This directed radiation is
then exposed to a nega-tively and a positively charged electrode. As a result, the
α-particles (positive charged particles) are directed towards the negative
charge, whereas the gamma rays are not affected by the charge at all.
Consequently, the negatively charged β-particles are directed towards the
positive charge.
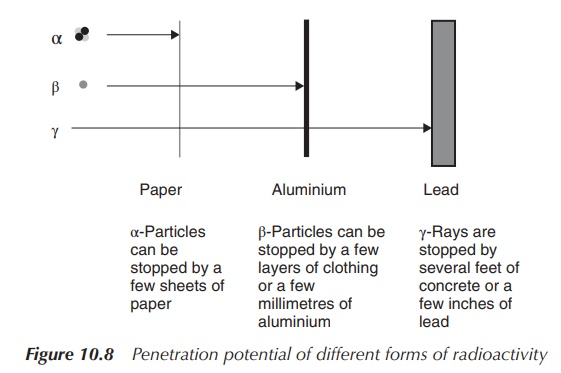
Penetration potential
It is important to understand the different penetration
potentials of the various forms of radioactive decay in order to evaluate their
clinical potential and the safety concerns of radiopharmaceutical compounds.
α-Particles have the shortest range and can be stopped by paper. Skin is
typically thick enough to provide sufficient protection. It is dangerous to
ingest α-particles as it can cause serious damage to the affected areas.
Unfortunately, it is difficult to monitor α-radiation. α-Particles have
typically a long half-life and cause cell death, and therefore can sometimes be
used in cancer treatment.
β-Particles cause ionisation and excitation (similar to
α-particles) when they interact with matter. Inges-tion is also a serious
problem, as many β-radioisotopes are isotopes of carbon, hydrogen, sulfur,
phosphorus and other essential elements and can easily be incorporated into
biological material in the human body. This can lead to extensive damage of DNA
and tissue. Additionally, many β-particles have relatively long half-lives.
Fortunately, they can be shielded by a few millimetres of aluminium or plastic
and are easy to monitor.
γ-Photons also cause ionisation and excitation, but not as
successfully as α-particles or β-particles. The energy of γ-photons is usually
larger than that of chemical bonds and can destroy biological structures in the
human body. Their penetration potential is the highest amongst the types of
radiation discussed here. γ-Photons can penetrate through skin and tissue
easily. Shielding requires a few inches of lead or a few feet of concrete.
Quantification of radioactivity
Radioactivity can be quantified, and there are several units
being used in order to describe the energy, exposure and the dose of radiation.
It is crucial for a nuclear pharmacist to understand these units in order to
dispense and handle radioactive material correctly.
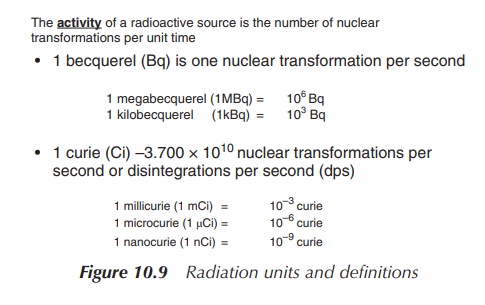
1. Units of radioactivity
The activity of a radioactive source is
defined as the number of transformations per unit time. The old tra-ditional
unit of radiation is curie (Ci). One curie is defined as 3.7 × 1010
disintegrations per second (dps). Nevertheless, the SI unit is becquerel (Bq),
which is equal to 1 dps and is a metric unit (Figure 10.8, Tables 10.1 and
10.2).
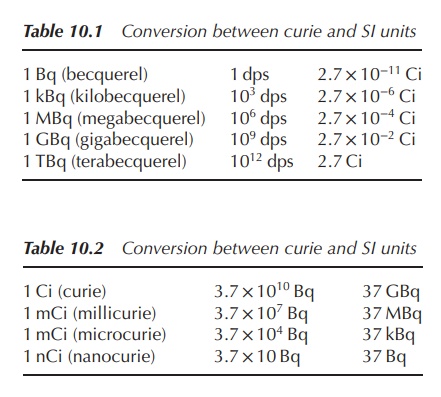
For a pharmacist, it is crucial to know the specific activity of
a radioactive preparation. This is the activity of a particular radionuclide
per unit mass of this element, usually expressed in grams. The radioactive
con-centration of a solution is defined as the activity of a particular
radionuclide per unit volume. The absorbed dose is the energy deposited per
unit mass of the material. The unit 1 gray (Gy) is equal to 1 J of energy
absorbed in 1 kg of material. As a comparison, ∼25 Gy is need to kill bacteria when sterilising. The ‘dose
equivalent’ takes also into account variations in the biological effectiveness
of different radiation. The unit is sievert (1 Sv) (Figure 10.9).
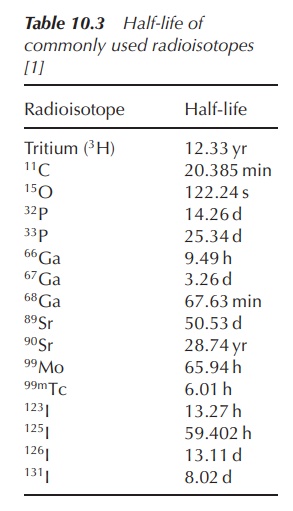
2. Half-life
(t1/2)
For nuclear pharmacists, it is also important to understand the term half-life (t1/2 ), as this gives information on how fast the radioactive decay takes place. The shorter the half-life, the faster the radioisotope decays.
The half-life of radioactive elements can vary
from several years to less than a second. Typical examples include 14C
(5730 years), 24Na (15 h) and 18Kr (13 s).
Radiopharmaceuticals are typically divided into products with long (>12 h) and short (<12 h)
half-lives.
Half-life (t1/2) is defined as the time it takes for the activity (or the amount of
radioactivity) to reduce by 50%. The
shorter the half-life, the faster the isotope decays and the more unstable it
is. The half-life is unique for any given radioisotope.
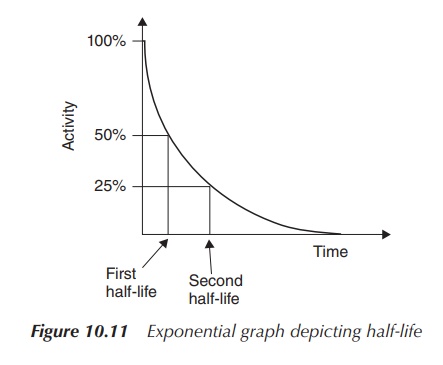
Radioactive decay follows an exponential curve and it is
therefore possible to determine the half-life by plotting a graph of activity
versus time (see Figure 10.10). The first half-life is the point on the time
axis at which only 50% of the initial activity remains. Subsequently, the
second half-life is the time point at which the activity has halved again, that
is, 25% of the original activity is left (Figure 10.11).
Dose equivalent = Absorbed dose × Quality factor
The quality factor for β- and γ-radiation is 1 and therefore 1 Sv = 1 Gy
Figure 10.10 Dose equivalent
The so-called decay constant λ is related to
half-life t1/2 and can be
calculated by using the following formula:
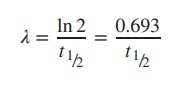
Example
The decay constant for 99mTc is
0.1153 h−1. Calculate the half-life (t1/2) of this radioisotope (Table 10.3).
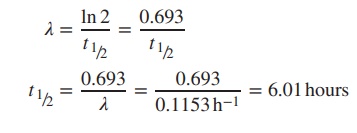
3. Calculation of radioactive decay
It is important for the healthcare
professional to predict the activity of the radioactive material at any point
in time before or after the assay being undertaken, as it is crucial to know
the exact activity at administration to the patient. The radioactive decay can
be described as the average number of radioactive isotopes (N) disin-tegrating per unit time
(=disintegration rate). The disintegration rate is defined as −dN/dt.
The disintegration rate is proportional to the number of undisposed
radioisotopes, and can be also expressed as the activity (A).
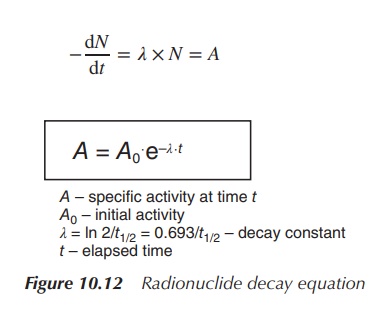
Upon integration, the radioactive decay of any radioactive
sample can be calculated by applying the so-called radionuclide decay equation
(see Figure 10.12). In order to calculate the radioactivity at a specific time
point t, it is important to know the
initial activity A0, the
elapsed time t and the decay constant
. Half-life is the time that passes
by until the activity has halved.
Example
A
radioactive sample has a half-life of 8.05 days and contains 150 mCi
radioactivity. Calculate the radioac-tivity left after 20 days.
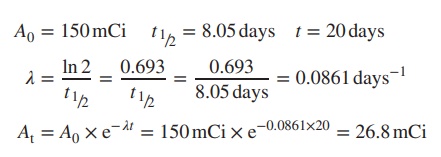
4. Dispensing of radioisotopes: percentage activity and activity concentration
For the dispensing pharmacist, it is crucial to know the
activity of a radioactive sample at any given time. The factor e− t from the decay equation A = A0e−
t is called the decay factor and can be used to
calculate the percentage activity remaining after given time t. The percentage activity can be
calculated using the following equation:
Percentage activity = 100 × e− λt
Example
131I has a half-life of 8.05 days. Calculate the percentage of 131I
remaining after 4.5 days.
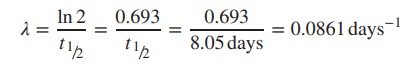
Percentage activity after 4.5 days = 100 × e− λt = 100 × e−0.0861×4.5 = 67.88%
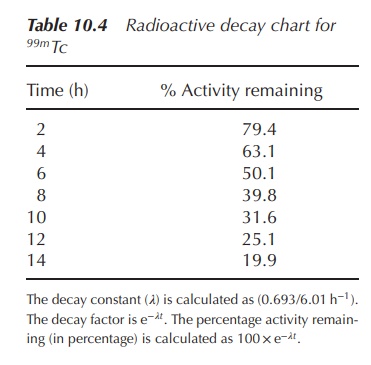
Radioactive decay charts, that is, charts showing the percentage
activity at certain time points, are often used in hospitals for quick
reference. A representative table illustrating the percentage activity for the
radioisotope 99mTc is shown in Table 10.4.
Radioisotopes are often dispensed according to their specific activity and activity concentration. The specific concentration is defined as the amount of radioactivity, usually expressed in curie, per unit mass. For example, a sample of containing 50 mg of 99mTc-albumin has an activity of 100 mCi. Therefore, the specific activity can be determined as 100 mCi/50 mg = 2 mCi/mg.
Most radioactive products are dispensed as
solutions, and therefore the term activity
concentration is important to understand. The activity concentration is
defined as the radioactivity expressed in curie, per unit volume. A 5 ml
solution of 99mTc-albumin has a radioactivity of 100 mCi. Therefore,
the activity con-centration is 100 mCi/5 ml = 20 mCi/ml. Once the activity
concentration is known, the right dose (volume) containing the correct
radioactivity can be dispensed.
Quantity(ml) = Activity required / Activity
concentration
Example
The
current activity concentration of a radiopharmaceutical is 20 mCi/ml. A
solution with a dose of 15 mCi has been ordered. What quantity needs to be
dispensed in order to provide a sample with the correct radioactivity?
activity concentration ∶ 20 mCi∕ml activity
requested ∶ 15
mCi
Quantity needed = 15 mCi∕20 mCi∕ml = 0.75 ml = amount to be dispensed
Related Topics
