Vitamin A
| Home | | Biochemistry |Chapter: Biochemistry : Vitamins
The retinoids, a family of molecules that are related to dietary retinol (vitamin A), are essential for vision, reproduction, growth, and maintenance of epithelial tissues.
VITAMIN A
The retinoids, a family of molecules that are related to dietary retinol (vitamin A), are essential for vision, reproduction, growth, and maintenance of epithelial tissues. They also play a role in immune function. Retinoic acid, derived from oxidation of retinol, mediates most of the actions of the retinoids, except for vision, which depends on retinal, the aldehyde derivative of retinol.
A. Structure of vitamin A
Vitamin A is often used
as a collective term for several related biologically active molecules (Figure
28.18). The term retinoids includes both natural and synthetic forms of vitamin
A that may or may not show vitamin A activity.
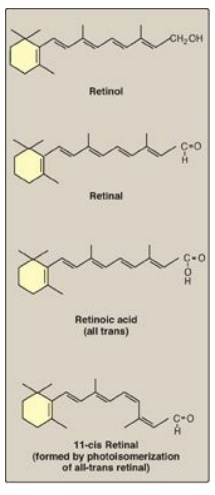
Figure 28.18 Structure of the retinoids.
1. Retinol: A primary alcohol containing a β-ionone ring with
an unsaturated side chain, retinol is found in animal tissues as a retinyl
ester with long-chain FAs.
2. Retinal: This is the aldehyde derived from the oxidation of
retinol. Retinal and retinol can readily be interconverted.
3. Retinoic acid: This is the acid derived from the oxidation of
retinal. Retinoic acid cannot be reduced in the body, and, therefore, cannot
give rise to either retinal or retinol.
4. β-Carotene: Plant foods contain β-carotene, which can be
oxidatively cleaved in the intestine to yield two molecules of retinal. In
humans, the conversion is inefficient, and the vitamin A activity of β-carotene
is only about 1/12 that of retinol.
B. Absorption and transport of vitamin A
1. Transport to the liver: Retinyl esters present in the diet
are hydrolyzed in the intestinal mucosa, releasing retinol and FFAs (Figure
28.19). Retinol derived from esters and from the cleavage and reduction of
carotenes is re-esterified to long-chain FAs in the intestinal mucosa and
secreted as a component of chylomicrons into the lymphatic system (see Figure
28.19). Retinyl esters contained in chylomicron remnants are taken up by, and
stored in, the liver.
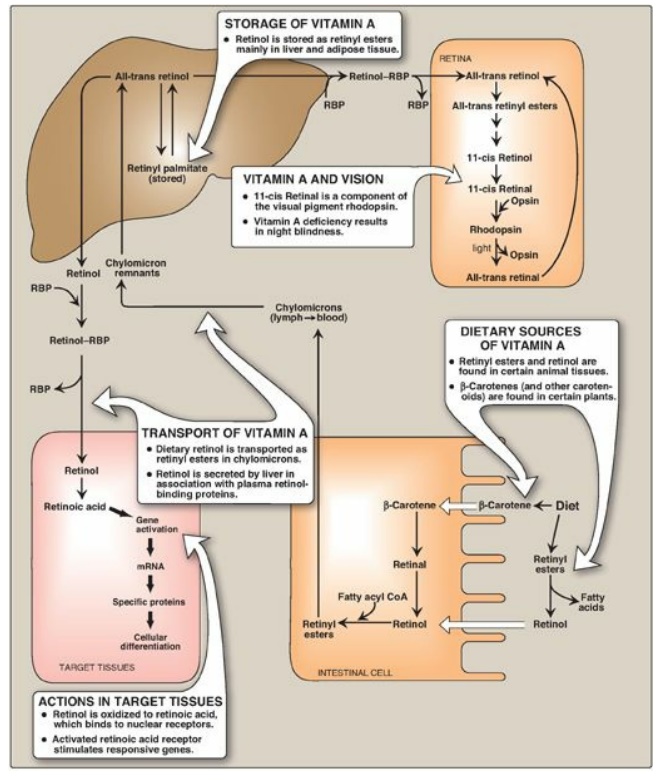
Figure 28.19 Absorption, transport, and storage of vitamin A and its derivatives. RBP = retinol-binding protein; CoA = coenzyme A; mRNA = messenger RNA.
2. Release from the liver: When needed, retinol is released
from the liver and transported to extrahepatic tissues by the plasma
retinol-binding protein (RBP). The retinol–RBP complex binds to a transport
protein on the surface of the cells of peripheral tissues, permitting retinol
to enter. Many tissues contain a cellular retinol-binding protein that carries
retinol to sites in the nucleus where the vitamin acts in a manner analogous to
that of steroid hormones.
C. Mechanism of action of vitamin A
Retinol is oxidized to retinoic acid. Retinoic acid binds with high affinity to specific receptor proteins (retinoic acid receptors [RARs]) present in the nucleus of target tissues such as epithelial cells (Figure 28.20). The activated retinoic acid–RAR complex binds to response elements on DNA and recruits activators or repressors to regulate retinoid-specific RNA synthesis, resulting in control of the production of specific proteins that mediate several physiologic functions. For example, retinoids control the expression of the gene for keratin in most epithelial tissues of the body. The RAR proteins are part of the superfamily of transcriptional regulators that includes the nuclear receptors for steroid and thyroid hormones and 1,25-dihydroxycholecalciferol, all of which function in a similar way.
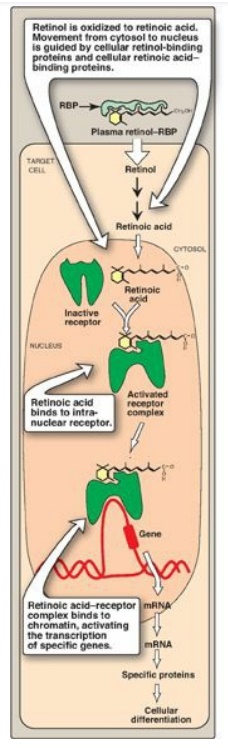
Figure 28.20 Action of the retinoids. [Note: Retinoic acid-receptor complex forms a dimer, but is shown as monomer for simplicity.] RBP = retinol-binding protein; mRNA = messenger RNA.
D. Functions of vitamin A
1. Visual cycle: Vitamin A is a component of the visual pigments of
rod and cone cells. Rhodopsin, the visual pigment of the rod cells in the
retina, consists of 11-cis retinal specifically bound to the protein opsin.
When rhodopsin is exposed to light, a series of photochemical isomerizations
occurs, which results in the bleaching of the visual pigment and release of
all-trans retinal and opsin. This process triggers a nerve impulse that is
transmitted by the optic nerve to the brain. Regeneration of rhodopsin requires
isomerization of all-trans retinal back to 11-cis retinal. All-trans retinal,
after being released from rhodopsin, is reduced to all-trans retinol,
esterfied, and isomerized to 11-cis retinol that is oxidized to 11-cis retinal.
The latter combines with opsin to form rhodopsin, thus completing the cycle.
Similar reactions are responsible for color vision in the cone cells.
2. Maintenance of epithelial cells: Vitamin A is essential for normal
differentiation of epithelial tissues and mucus secretion, and thus, supports
the body’s barrier-based defense against pathogens.
3. Reproduction: Retinol and retinal are essential for normal reproduction, supporting spermatogenesis in the male and preventing fetal resorption in the female. Retinoic acid is inactive in maintaining reproduction and in the visual cycle but promotes growth and differentiation of epithelial cells. Therefore, animals given vitamin A only as retinoic acid from birth are blind and sterile.
E. Distribution of vitamin A
Liver, kidney, cream,
butter, and egg yolk are good sources of preformed vitamin A. Yellow, orange,
and dark green vegetables and fruits are good dietary sources of the carotenes,
which serve as precursors of vitamin A.
F. Requirement for vitamin A
The RDA for adults is
900 retinol activity equivalents (RAEs) for males and 700 RAE for females. In
comparison, 1 RAE = 1 mg of retinol, 12 mg of β-carotene, or 24 mg of other
carotenoids.
G. Clinical indications
Although chemically
related, retinoic acid and retinol have distinctly different therapeutic
applications. Retinol and its carotenoid precursor are used as dietary
supplements, whereas various forms of retinoic acid are useful in dermatology.
1. Dietary deficiency: Vitamin A, administered as retinol or retinyl esters, is used to treat patients who are deficient in the vitamin (Figure 28.21). Night blindness is one of the earliest signs of vitamin A deficiency. The visual threshold is increased, making it difficult to see in dim light. Prolonged deficiency leads to an irreversible loss in the number of visual cells. Severe vitamin A deficiency leads to xerophthalmia, a pathologic dryness of the conjunctiva and cornea, caused, in part, by increased keratin synthesis. If untreated, xerophthalmia results in corneal ulceration and, ultimately, in blindness because of the formation of opaque scar tissue. The condition is most commonly seen in children in developing tropical countries. Over 500,000 children worldwide are blinded each year by xerophthalmia caused by insufficient vitamin A in the diet.
2. Acne and psoriasis: Dermatologic problems such as acne
and psoriasis are effectively treated with retinoic acid or its derivatives
(see Figure 28.21). Mild cases of acne, Darier disease (keratosis
follicularis), and skin aging are treated with topical application of tretinoin
(all-trans retinoic acid), as well as benzoyl peroxide and antibiotics. [Note:
Tretinoin is too toxic for systemic administration and is confined to topical
application.] In patients with severe cystic acne unresponsive to conventional therapies, the drug of choice is
isotretinoin (13-cis retinoic acid) administered orally. Retinoic acid is also
used in the treatment of acute promyelocytic leukemia.
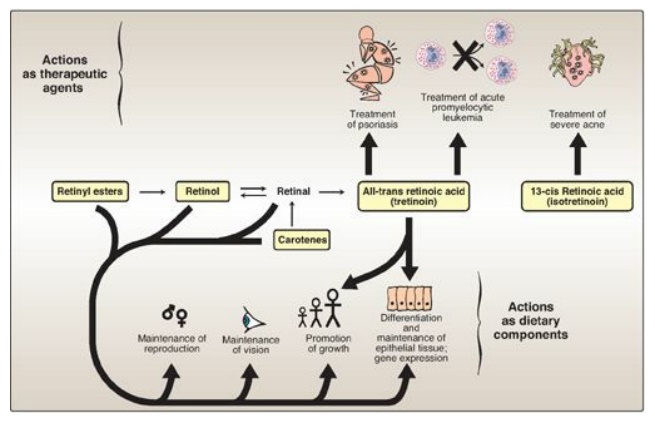
Figure 28.21 Summary of actions of retinoids. Compounds in boxes are available as dietary components or as pharmacologic agents.
H. Toxicity of retinoids
1. Vitamin A: Excessive intake of vitamin A produces a toxic
syndrome called hypervitaminosis A. Amounts exceeding 7.5 mg/day of retinol
should be avoided. Early signs of chronic hypervitaminosis A are reflected in
the skin, which becomes dry and pruritic (due to decreased keratin synthesis);
the liver, which becomes enlarged and can become cirrhotic; and in the CNS,
where a rise in intracranial pressure may mimic the symptoms of a brain tumor.
Pregnant women particularly should not ingest excessive quantities of vitamin A
because of its potential for teratogenesis (causing congenital malformations in
the developing fetus). UL is 3,000 mg/day. [Note: Vitamin A promotes bone
growth. In excess, however, it is associated with decreased bone mineral
density and increased risk of fractures.]
2. Isotretinoin: The drug, an isomer of retinoic acid, is teratogenic and absolutely contraindicated in women with childbearing potential unless they have severe, disfiguring cystic acne that is unresponsive to standard therapies. Pregnancy must be excluded before initiation of treatment, and adequate birth control must be used. Prolonged treatment with isotretinoin leads to hyperlipidemia with an increase in TAGs and cholesterol, providing some concern for an increased risk of cardiovascular disease.
