Type B or Idiosyncratic Adverse Drug Reactions
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Mechanisms of Adverse Drug Reactions
Idiosyncratic adverse reactions are less common than the pharmacological adverse reactions but are as important, if not more so, because they are often more serious and account for many drug-induced deaths.
TYPE B OR IDIOSYNCRATIC ADVERSE
DRUG REACTIONS
Idiosyncratic
adverse reactions are less common than the pharmacological adverse reactions
but are as important, if not more so, because they are often more serious and
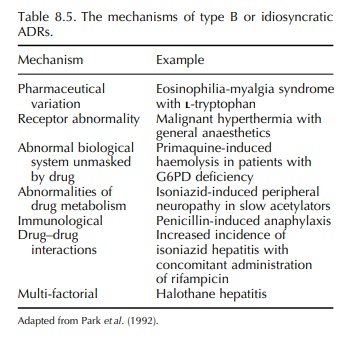
account for many drug-induced deaths. The possible mechanisms of idiosyncratic
adverse effects (Park, Pirmohamed and Kitteringham, 1992) are listed in Table
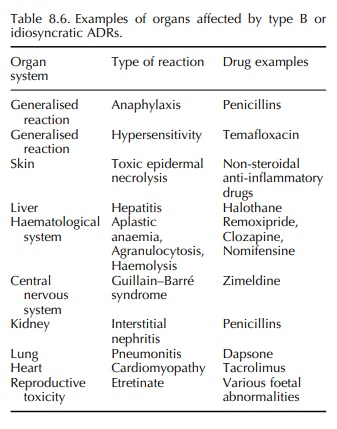
8.5. The toxic reactions may affect many organ systems either in isolation or
in combi-nation (Table 8.6).
Type B ADRs have been characterised as being dose-independent (Table 8.2) or rather there is no simple relationship between dose and the occur-rence of toxicity (Park, Pirmohamed and Kittering-ham, 1998). Certainly, the evaluation of patients with and without hypersensitivity to a particular compound shows very little difference in doses received, and indeed in the patients with hypersensitivity, the doses may have been lower because the drug had to be withdrawn. Furthermore, even within the hypersensi-tive group, there is little relationship to the occurrence and severity of toxicity and the dose administered. However intuitively, there must be some kind of dose-response relationship because if the patient had not received the drug, then they would not have developed the hypersensitivity reaction. Because many type B ADRs are thought to be mediated by the formation of chemically reactive metabolites (CRMs) through metabolism by P450 enzymes (a process termed ‘bioactivation’) (Park, Pirmohamed and Kitteringham, 1998), perhaps a relationship exists with the ‘internal dose’, i.e. the concentration of the toxic metabolite formed in the body. However, because these metabo-lites by definition are unstable, it has not been possible with the currently available technologies to evaluate the dose-response relationship. The situation is further compounded by the fact that the different sources of variation in the human body may all have a different dose-response relationship. Nevertheless, evidence for the existence of such a dose-response relationship can be gleaned from clinical situations where different doses have to be given to the same group of patient in different circumstances. For example, in HIV-positive patients, the anti-infective agent co-trimoxazole has to be given at low doses for prophylaxis against Pneumocystis carinii pneumonia (PCP) (960 mg once daily), whereas for acute treatment of PCP, much higher doses (up to 8 g/day) may be administered. The frequency of hypersensitivity reactions is lower with the prophylactic dose (30%) than with the acute dose, where rates as high as 80% have been reported (Carr and Cooper, 1995; Pirmohamed and Park, 1995).
Related Topics
