The Need for Antimicrobial Stewardship
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotic Prescribing And Antibiotic Stewardship
In testimony to a US government committee in June 2010, the Infectious Diseases Society of America (IDSA) stated that Most commonly used antibiotics cost only a few dollars for a typical course of treatment… (and) a single course of antibiotics has the potential to protect and preserve many quality years of life for many people. No other type of medicine can claim such an achievement at such a price.
ANTIBIOTIC
PRESCRIBING AND ANTIBIOTIC STEWARDSHIP
THE NEED FOR ANTIMICROBIAL STEWARDSHIP
In testimony to a US government committee in June 2010, the Infectious
Diseases Society of America (IDSA) stated that Most commonly used
antibiotics cost only a few dollars for a typical course of treatment… (and) a
single course of antibiotics has the potential to protect and preserve many
quality years of life for many people. No other type of medicine can claim such
an achievement at such a price.
These statements were made as part of the argument presented by the IDSA
to promote both antibiotic research and appropriate use (‘stewardship’) of
antibiotics.
The wider point being made was that antibiotics have, since their
discovery in the 1940s, revolutionized medicine, and many of the procedures
that are taken for granted now —transplantation, cancer treatment, the care of
premature babies and several forms of surgery—would be impossible without them.
Yet, unfortunately, largely because they have been taken for granted, the
antibiotics we already possess are becoming less effective as a result of
bacterial resistance, and the prospects for producing new antibiotics currently
look bleak. It was recognized right from the start of the antibiotic era that
bacteria had the potential to develop resistance to antimicrobial drugs, but it
was quite some time before the perception of antibiotic resistance changed from
one in which it was regarded as unusual to one where it was expected; in other
words, a recognition that long-term efficacy was the exception, and resistance
was the rule.
As the 20th century drew to a close the
emergence of the antibiotic-resistant pathogens brought with it both the
spectre of untreatable infections where the organisms responsible were
resistant to all available agents and a growing sense of urgency to take steps
to preserve the usefulness of the antibiotics we currently have. The situation
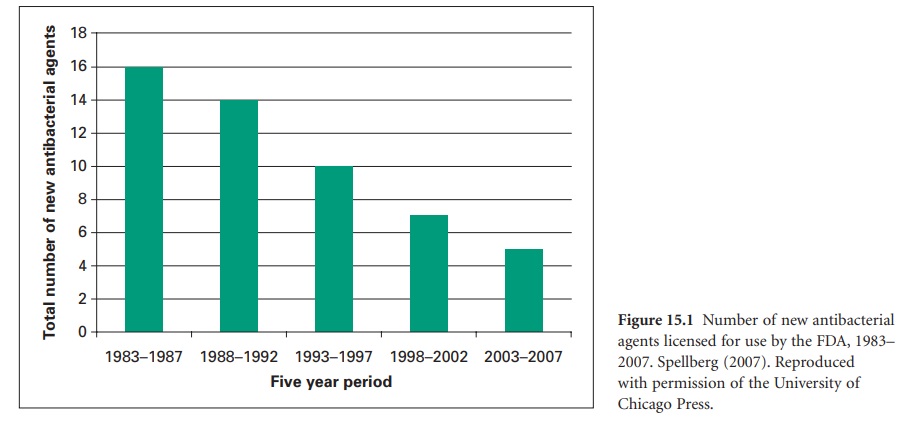
was exacerbated by a reduction in the number of new antibiotics coming into clinical
use. Figure 15.1 shows
that this number has continued to decline steadily for the last 20 years—a
trend that would be difficult to reverse in the short term simply because
several of the major international pharmaceutical companies are moving out of
antimicrobials as a research and development area. Unfortunately, antibiotics
have, in a sense, become victims of their own success: the more effective an
antimicrobial is, the shorter the likely duration of treatment, so the lower
the payback to the company that developed it. Many courses of antibiotic
treatment last for a week or less, so the sales accruing from them are far
inferior to those from drugs treating chronic conditions like diabetes and hypertension.
This fact, together with (1) increasing pressure to use antibiotics sparingly
anyway, (2) the expectation that the drug will ultimately become less effective
due to resistance and (3) difficulties in establishing clinical trials for
antibiotics that satisfy the US Food and Drugs Administration (FDA) criteria,
all combined to create a climate in which antibiotics became an unattractive
commercial proposition (except in the case of HIV/AIDS therapies that have to
be taken throughout the patient’ s life).
Certainly the problem of growing
antibiotic resistance had been recognized for some time before the end of the
20th century and policies designed to improve the quality of antibiotic prescribing
and restrict resistance in hospitals became progressively more common in Europe
and North America from the 1970s onwards. However, the increasing frequency in
the new millennium of infections due to the so-called ‘ESKAPE’ pathogens( Enterococcus faecium, Staphylococcus aureus, Klebsiella species,
Acinetobacter baumannii, Pseudomonas aeruginosa and extended
spectrum β-lactamase-producing strains of Escherichia coli and Enterobacter species) together with the drying up
of the pipeline of new antibiotics from the pharmaceutical industry has further
increased the tempo of measures to preserve what is increasingly being seen as
a precious, and perhaps irreplaceable, resource that society has a duty to pass
on to future generations rather than squander. These measures include the ‘10 ×
20’ initiative to establish an international research effort to develop 10 new
antibiotics by the year 2020, and the STAAR (Strategies to Address Antimicrobial
Resistance) Act, which is, at the time of writing, being considered by the US
Congress.
The cost of antibiotic resistance
should, of course, be measured primarily in terms of the suffering that results
from the failure of antibiotics to cure infections against which they were
formerly effective. The antibiotic-resistant pathogens responsible for these
infections can arise both in the home or the hospital environment, but it is in
the latter that they are, by far, more common and problematic. Hospital-acquired
infections account for a substantial number of deaths each year and the treatment
of such infections is time-consuming, difficult and costly. In its testimony to
Congress as part of the consultation process accompanying the STAAR Act, the
IDSA stated that antibiotic-resistant infections acquired within hospitals were responsible for 90 000
deaths each year in the USA and cost the healthcare system between $21 and $34
billion annually. There are less obvious consequences of resistance too: when
the first-line drugs cease to be effective it is sometimes necessary to revert
to alternatives that are more toxic. Acinetobacter infections
are a good example of this situation because the organism is naturally
multidrug resistant and the incidence of isolates resistant to all first-line
antibiotics has risen in the USA from 5% to 40% in 10 years, so now colistin, a
drug that became virtually obsolete in the 1960s because of the significant
risk of kidney damage is, for many patients, the most likely antibiotic choice.
There is widespread agreement that
greater use of antibiotics predisposes to the development of resistance. The
strength of the link between use and resistance varies from one antibiotic to
another, but for many antibiotics the connection is irrefutable. However, the
situation is far from simple: there is substantial evidence, for example, that
heavy use of one antibiotic may be a risk factor for the acquisition of
infections by organisms resistant to other, unrelated antibiotics—heavy
cephalosporin use has been shown to increase the risk of vancomycin resistant
enterococci, and fluoroquinolone use has been associated with the prevalence of
meticillin-resistant Staph. aureus (MRSA).
The selective pressure created by the use of one antibiotic will often select
for resistance in others because plasmids within the bacterial cell may carry
resistance genes for multiple antibiotics from different chemical groups. If, for
example, an organism possessed a plasmid with genes for both rifampicin and
gentamicin resistance, constant exposure to gentamicin would represent a
selective pressure that afforded an advantage to that organism so, not only
would the incidence of isolates with gentamicin resistance be expected to rise,
but so too would the incidence of rifampicin resistant isolates.
Arguments for curtailing antibiotic use in order to restrict resistance
development have been supported by audits and surveys of antibiotic prescribing
and costs. It has been estimated that up to 50% of antibiotic prescribing is either
inappropriate (wrong drug, duration, dose, etc.) or unnecessary (not required at
all). Antibiotic consumption varies widely throughout Europe: a 2005 survey showed
that France, for example, used three times as much antibiotics per head of
population as the Netherlands, but this difference was not justified by higher
infection rates or better cure rates, so logic suggests that part of the
antibiotic use in France was unnecessary. Inappropriate prescribing and
consumption, together with the fact that antibiotics can represent up to 30% of
a hospital pharmacy budget, have provided further impetus for measures designed
to achieve more prudent prescribing. Such thinking is not new, however; it was
the rationale for the introduction of antibiotic policies in the 1970s and
1980s which, in addition to setting out the general standards for safe and
appropriate prescribing, advised on the selection of antibiotics for specific
infections, for special situations like surgical prophylaxis and for the
treatment of specific groups of patients, e.g. the newborn, those with poor
kidney function and the immuno-compromised. What has changed since then is the
recognition of the need for a much broader approach to the problem —in other
words, the need for a comprehensive antimicrobial stewardship strategy that
incorporates, but also extends, the policies formulated in the last century.
There is not a universally accepted definition of, or agreement upon,
what constitutes an antimicrobial stewardship programme. For most people the
term applies particularly, or even exclusively, to the manner in which
antibiotics are used and distributed in hospitals. However, some see it in a
much broader sense as a range of initiatives which, together, impact upon
antibiotic resistance but are not necessarily even confined to
antibiotic-related practices in the hospital or the home. The great majority of
the annual global production of antibiotics is not used in the treatment of
human or animal infection anyway. Most of the antibiotic output of the
international pharmaceutical industry is used as a food additive to increase
weight gain in cattle, pigs and poultry —some estimates put this proportion as
high as 80%—and yet more is used in plant production, but this fraction is
ill-defined. Although antibiotics that are used to treat human infections have
been banned as growth promoters in Europe for many years it is still a common
practice in many countries, and even the legitimate veterinary use of
antibiotics is considerable: the total volume of antibiotics used in the UK for
agricultural purposes in 2007 was 387 tonnes. Curtailment of the use of
antibiotics for growth promotion—which many see as inappropriate and a likely
contributor to resistance development—together with better-targeted and-promoted
use of vaccines (that would reduce the need for antibiotics), better diagnostic
agents which would more rapidly and accurately identify the infecting organism and
so inform the selection of the best antibiotic, better epidemiological data and
computer analysis to provide early warnings of resistance trends, and changes
in other medical practices like the early removal of catheters and cannulas
which are, themselves, a means by which pathogens can enter the body, might all
be seen as part of a stewardship programme. But from the perspective of
controlling the incidence and spread of antibiotic-resistant organisms within a
hospital it cannot be over-emphasized that a comprehensive infection control
programme is of paramount importance and the best results are achieved when
data from antibiotic stewardship and infection control can be linked and
analysed together.
It is generally accepted that the principal goals of a stewardship
programme are to:
• Improve patient outcomes
• Lessen the risk of adverse effects
• Reduce resistance levels, or at least slow the rate of resistance
development
• Improve cost-effectiveness.
Following the international financial crisis that began in 2007 it is
likely, at least in many European countries, that the last of these goals will
receive particular attention, so it is worth emphasizing that stewardship programmes
can be self-financing. Although there is an initial start-up cost, there is
substantial evidence that this is rapidly recovered by cost savings resulting
from reductions in antimicrobial use that some reports have estimated to vary
from 22 to 36%.
Related Topics
