Structure of Fatty Acids
| Home | | Biochemistry |Chapter: Biochemistry : Fatty Acid, Ketone Body, and Triacylglycerol Metabolism
A fatty acid consists of a hydrophobic hydrocarbon chain with a terminal carboxyl group that has a pKa of about 4.8.
STRUCTURE OF FATTY ACIDS
A fatty acid consists
of a hydrophobic hydrocarbon chain with a terminal carboxyl group that has a
pKa of about 4.8 (Figure 16.2). At physiologic pH, the terminal carboxyl group
(–COOH) ionizes, becoming –COO–. [Note: When the pH is above the pK, the
deprotonated form predominates.] This anionic group has an affinity for water,
giving the fatty acid its amphipathic nature (having both a hydrophilic and a
hydrophobic region). However, for long-chain fatty acids (LCFAs), the
hydrophobic portion is predominant. These molecules are highly water insoluble
and must be transported in the circulation in association with protein. More
than 90% of the fatty acids found in plasma are in the form of fatty acid esters
(primarily TAG, cholesteryl esters, and phospholipids) contained in circulating
lipoprotein particles. Unesterified (free) fatty acids are transported in the
circulation in association with albumin, the most abundant protein in serum.

Figure 16.2 Structure of a
fatty acid.
A. Saturation of fatty acids
Fatty acid chains may
contain no double bonds (that is, be saturated) or contain one or more double
bonds (that is, be mono- or polyunsaturated). In humans, the majority are
saturated or monounsaturated. When double bonds are present, they are nearly
always in the cis rather than in the trans configuration. (See : for a
discussion of the dietary occurrence of cis and trans unsaturated fatty acids.)
The introduction of a cis double bond causes the fatty acid to bend or “kink”
at that position (Figure 16.3). If the fatty acid has two or more double bonds,
they are always spaced at three-carbon intervals. [Note: In general, addition
of double bonds decreases the melting temperature (Tm) of a fatty
acid, whereas increasing the chain length increases the Tm. Because
membrane lipids typically contain LCFAs, the presence of double bonds in some
fatty acids helps maintain the fluid nature of those lipids.]
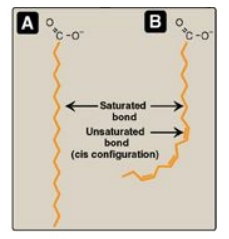
Figure 16.3 A saturated (A)
and an unsaturated (B) fatty acid. Orange denotes hydrophobic portions of the
molecules. [Note: Cis double bonds cause a fatty acid to “kink.”].
B. Chain lengths of fatty acids and positions of double bonds
The common names and structures
of some fatty acids of physiologic importance are listed in Figure 16.4. In
humans, fatty acids with an even number of carbon atoms (16, 18, or 20)
predominate, with longer fatty acids (over 22 carbons) being found in the
brain. The carbon atoms are numbered, beginning with the carboxyl carbon as carbon
1. The number before the colon indicates the number of carbons in the chain,
and those after the colon indicate the numbers and positions (relative to the
carboxyl end) of double bonds. For example, as denoted in Figure 16.4,
arachidonic acid, 20:4(5,8,11,14), is 20 carbons long and has 4 double bonds
(between carbons 5–6, 8– 9, 11–12, and 14–15). [Note: Carbon 2, the carbon to
which the carboxyl group is attached, is also called the α-carbon, carbon 3 is
the β-carbon, and carbon 4 is the γ-carbon. The carbon of the terminal methyl
group is called the ω-carbon regardless of the chain length.] The double bonds
in a fatty acid can also be denoted relative to the ω (or methyl) end of the
chain. Arachidonic acid is referred to as an ω-6 fatty acid acid (also an n-6
fatty acid, Figure 16.5A) because the terminal double bond is six bonds in from
the ω end (Figure 16.5B). Another ω-6 fatty acid is the essential linoleic acid
18:2(9,12). In contrast, α-linolenic acid, 18:3(9,12,15), is an essential ω-3
fatty acid. (See : for a discussion of the nutritional significance of ω-3 and
ω-6 fatty acids.)
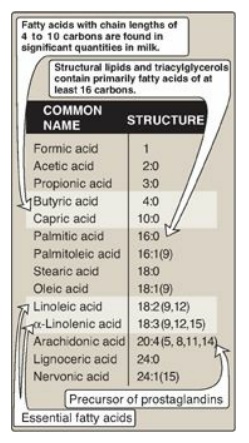
Figure 16.4 Some fatty acids
of physiologic importance. [Note: If a fatty acid has 2–4 carbons, it is
considered short; if 6–12, medium; if 14–20, long; and if 22 or more, very
long.].
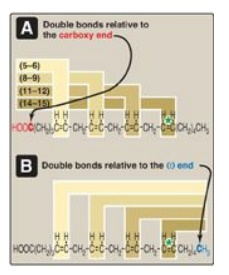
Figure 16.5 Arachidonic acid,
illustrating the position of the double bonds. Arachidonic acid,
20:4(5,8,11,14) is an n-6 fatty acid because the double bond furthest from the
carboxy end (carbon 1) is 14 carbons from that end: 20 - 14 = 6. It is also
referred to as an ω-6 fatty acid because the terminal double bond is six bonds
in from the ω end. Thus, the “ω” and “n” designations are equivalent (see *).
C. Essential fatty acids
Linoleic acid, the
precursor of ω-6 arachidonic acid, which is the substrate for prostaglandin
synthesis, and α-linolenic acid, the precursor of ω-3 fatty acids that are
important for growth and development, are dietary essentials in humans because
we lack the enzymes needed to synthesize them. Plants provide us with these
essential fatty acids. [Note: Arachidonic acid becomes essential if linoleic
acid is deficient in the diet.]
Essential fatty acid deficiency (rare) can result
in a dry, scaly dermatitis as a result of an inability to synthesize molecules
that provide the water barrier in skin.
Related Topics
