Steroid Nomenclature and Structure
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Steroids
Steroids consist of four fused rings (A, B, C, and D). Chemically, these hydrocarbons are cyclopentano per hydro phenenthrenes.
STEROID NOMENCLATURE AND STRUCTURE
Steroids
consist of four fused rings (A, B, C, and D). Chemically, these hydrocarbons
are cyclopentano per hydro phenenthrenes. They contain a five-membered
cyclopentane (D) ring and the three rings of phenanthrene. A perhydro
phenanthrene (ring A, B, and C) is the saturated derivative of phenanthrene.
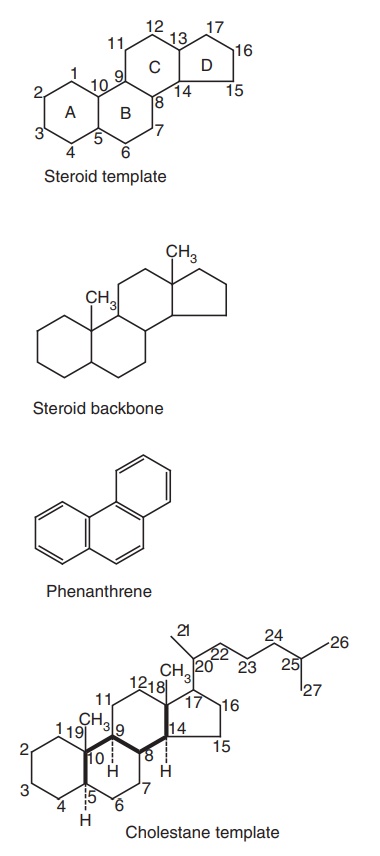
The
polycyclic hydrocarbon, known as 5α-cholestane, is used to illustrate the
numbering system for a steroid.
·The ring juncture or backbone carbons are shown
in the structure of 5α-cholestane with a heavy dark line.
·Solid lines denote groups above the plane of the
nucleus (β-configuration) and dotted or broken lines denote groups below the
plane (α-configuration). If the configuration of substituent is unknown, its bond
to the nucleus is drawn as a wavy line.
·The configuration of the H at C-5 is always
indicated in the name.
·Circles were sometimes used to indicate α-hydrogens
and dark dots to indicate β-hydrogens.
·Compounds with 5α-cholestane belong to
allo-series, while compounds derived from 5 β-cholestane belong to the normal
series.
·If the double bond is not between sequentially
numbered carbons, in such cases, both carbons are indicated in the same.
·When a methyl group is missing from the side
chain, this is indicated by the prefix ‘nor’ with the number of carbon atom,
which has disappeared.
The symbol Δ
is often used to designate a C = C bond in a steroid. If C = C is in between
carbons 5 and 4, the compound is referred to as a Δ4 steriod, and if
the C = C bond is between positions 5 and 10, the compound is designated as Δ5(10)
steroid. Example, Estra-1,3,5(10) triene-3,17b-diol.
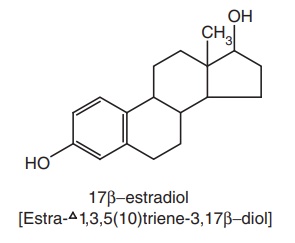
Since 17 β-estradiol
contains 18 carbon atoms, it is considered as a derivative of estrane, a basic
nucleus.

Stereochemistry: The absolute stereochemistry of the molecule and
any substituent is shown with solid (β) and dashed (α) bonds; a (axial) bond is
perpendicular to the plane of the molecule while equatorial bond (e) is horizontal to the plane of
the molecule.
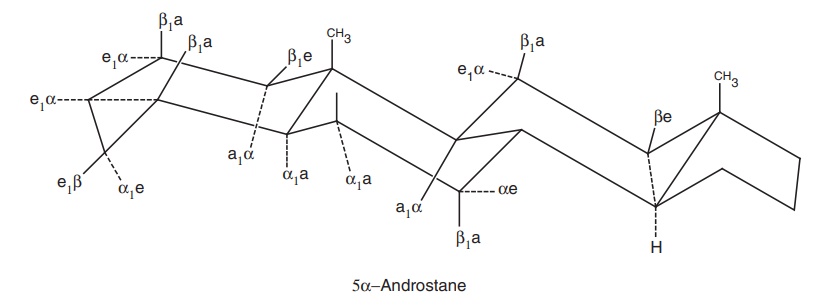
The
aliphatic side chain at position is always assumed to be of β-configuration.
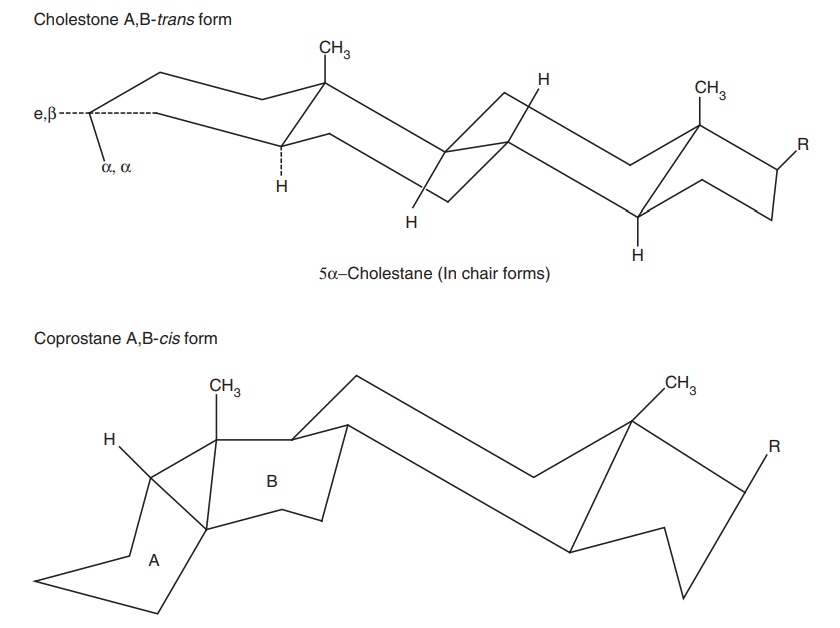
The term cis and trans are used occasionally to indicate the backbone
stereochemistry between rings. For example, 5 α steroids are A/B trans and 5 β-steroids are A/B cis. The terms syn and anti are used
analogously to trans and cis.
Conformations: There are six asymmetric carbon atoms in the
nucleus 5, 8, 9, 10, 13, and 14. Therefore, there are 26 = 64
optically active forms possible. Cholestane, androstane, and pregnane can exist
in two conformations, that is, chair form and boat form.
Chair
confirmation is more stable than boat confirmation due to less angle strain, and
hence, all cyclohexane rings in the steriod nucleus exist in the chair
confirmation.
Related Topics
