Sodium: an essential ion in the human body
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkali Metals
Sodium has atomic number 11 and has the symbol Na, derived from the Latin name ‘natrium’. Sodium ions (Na+) are soluble in water and therefore present in large quantities in the oceans. Na+ is also part of minerals and an essential element for all animal life.
Sodium:
an essential ion in the human body
Sodium has atomic number 11 and has the symbol
Na, derived from the Latin name ‘natrium’. Sodium ions (Na+) are
soluble in water and therefore present in large quantities in the oceans. Na+
is also part of minerals and an essential element for all animal life.
The main biological roles of sodium ions are
the maintenance of body fluids in humans and the functioning of neurons and
transmission of nerve impulses. Na+ is an important electrolyte and
a vital component of the extracellular fluid. Therefore, one of its roles is to
maintain the fluid in the human body via osmoregulation, a passive transport
mechanism (Section 2.3.1). Na+ ions also play a crucial role in the
contraction of muscles and in the mode of action of several enzymes. In the
human body, Na+ is often used to actively build up an electrostatic
potential across membranes, with potassium ions (K+) being the
counter-ion (Section 2.3.2). The build-up of an electrostatic potential across
cell membranes is important to allow the transmission of nerve impulses.
1. Osmosis
Osmosis is defined as the physical process of diffusion of a solvent
(water) through a semi-permeable membrane
towards an area of high solute (salt) concentration. This means that solvent
(water) follows the osmotic gradient by moving across the semi-permeable
membrane from one solution where there is a lower salt concentration towards a
second solution with a high salt concentration in order to dilute this and to
equalise the concentrations.
Sodium is an essential mineral for the human body and crucial
for the regulation of the body fluid via its osmosis activity. Sodium ions
account for over 90% of all ions in the plasma and in the interstitial fluid,
which are involved in osmosis processes. Furthermore, it is the most abundant
cation in the extracellular fluid, and therefore the Na+ content
controls the extracellular volume. In particular, the kidneys play an important
role in regulating the fluid level of the body as well as the filtration,
secretion and re-absorption of Na+ in the nephrons, the functional
unit of the kidney. Na+ ions are used in the human body to establish
osmotic gradients, which in turn is crucial to control the water balance.
Furthermore, decreases in blood pressure and in Na+ concentrations
are sensed by the kidneys, and hormones (e.g. renin, antidiuretic hormones
(ADHs), atrial natriuretic peptide) are released that control the blood
pressure, osmotic balances and water-retaining mechanisms.
In general, if a medium is
•
hypertonic, that
means the solution has a higher concentration of solutes than the surrounding
area. This area will lose water
through osmosis;
•
isotonic, that
means the solution has the same concentration of solutes as the surrounding
area. No move-ment of water will occur;
•
hypotonic, that
means the solution has a lower concentration of solutes than the surrounding
area. This area will gain water
through osmosis (Figure 2.12).
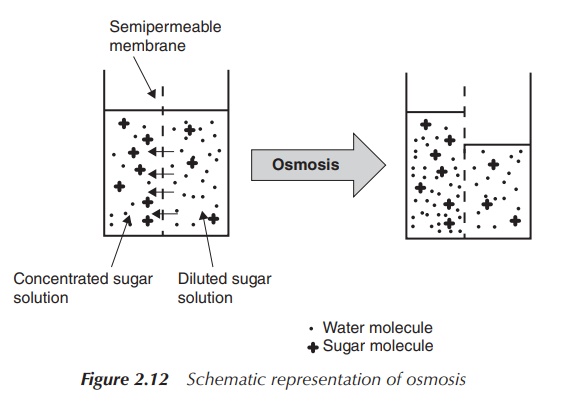
A net movement of the solvent (water) occurs from the hypotonic
solution to the solution with the higher concentration in order to reduce the
difference in concentrations. The osmotic pressure is defined as the pressure
that is required to establish equilibrium with no movement of solvents. It is
important to mention that osmotic pressure depends on the number of ions or
molecules in the solution, not the identity of those. The unit often being
found to describe the osmotic pressure is the osmole (osmol or osm), which is a
non-SI unit that defines the numbers of moles of a compound that contributes to
the osmotic pressure of a solution.
In general, osmotic processes are important for many biological processes. Plants use osmosis to transport water and solutes through their systems and the osmotic gradient to establish the turgor within cells. The human body uses osmosis for many processes, the excretion of urine being one of the most prominent one.
Urine production takes place within the kidney, more
specifically at the nephrons which are the functional units of the kidneys.
Approximately 150–180 of plasma is filtered every day through the glomerulus,
which is a part of the nephron, in order to produce the urine. The nephron also
consists of the proximal tubule, the Loop of Henle and the distal tubule, which
leads to the collecting duct and ultimately to the ureter (Figure 2.13) .
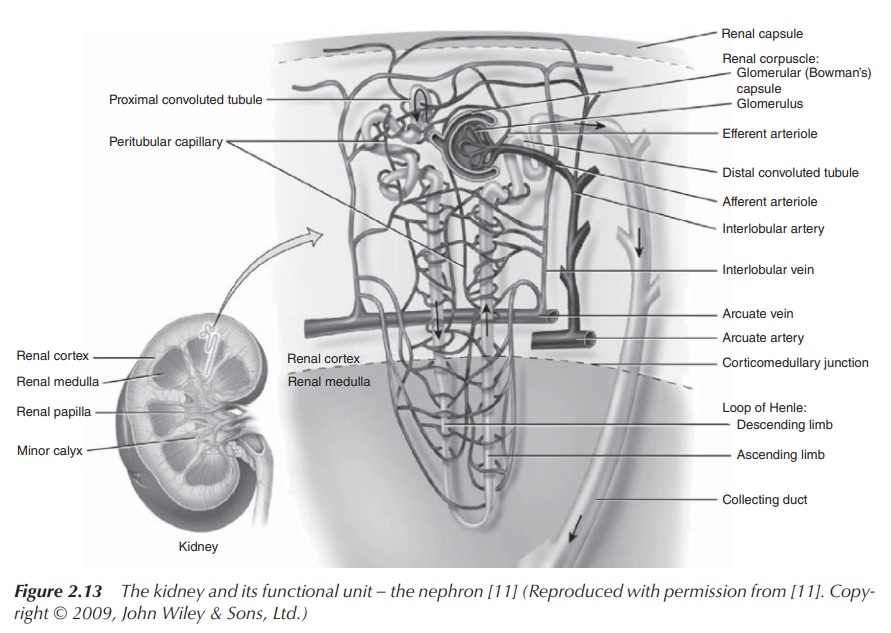
Filtration takes places at the glomerulus,
whereas the remaining parts of the nephron are responsible for the secretion
and re-absorption of ions in order to regulate imbalances and manage the urine
volume before the urine is stored in the bladder. This secretion and re-absorption
can occur via an active or a passive transport across the nephron membrane. Na+
is usually actively transported across via Na+ pumps in order to
establish the correct Na+ concentration in the blood plasma, which
is responsible for maintaining the correct osmotic pressure. Via this process,
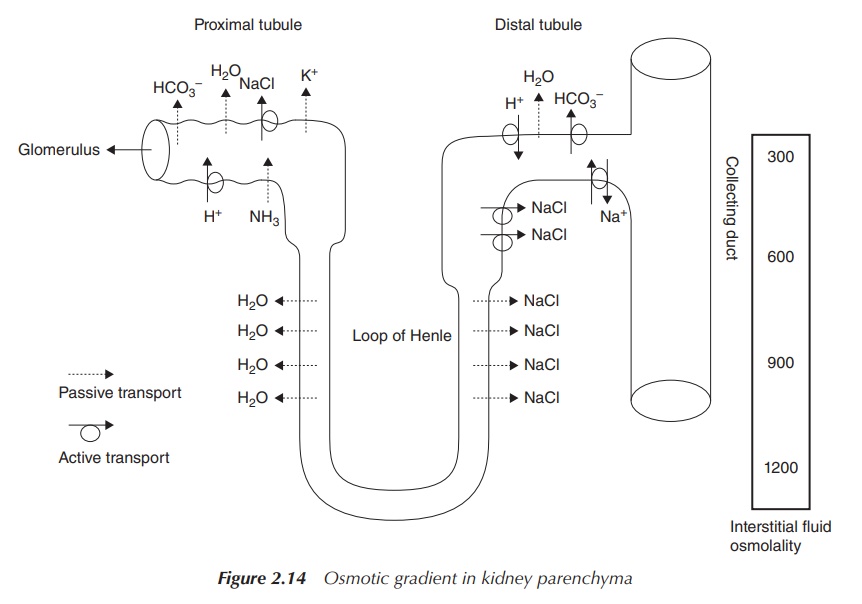
an osmotic gradient is established within the kidney parenchyma, which is used
to conserve water. The ascending limb of the Loop of Henle is impermeable to
water but permeable to Na+. As a result, an osmotic gradient is
established. The descending limb of the Loop of Henle is permeable to water
and, as a result of the osmotic gradient, water moves to the interstitial fluid
and urine is concentrated. The collecting ducts can be permeable to water if
the body sends out a signal that water has to be conserved. Again, water will
passively follow the osmotic gradient and urine will be concentrated even more
(Figure 2.14).
2. Active
transport of sodium ions
As previously mentioned, the active transport of sodium ions is crucial for the functioning of, for example, neurons and the subsequent transmission of a nerve impulse. This can be achieved by the active build-up of a concentration gradient along the cell membrane using Na+/K+ pumps as the active unit. This active transport is responsible for the cells containing relatively high concentrations of potassium ions and low concentrations of sodium ions.
The resulting electrostatic potential that is built up along the cell membrane
is called action potential and is
subsequently responsible for the transmission of nerve impulses (Figure 2.15).
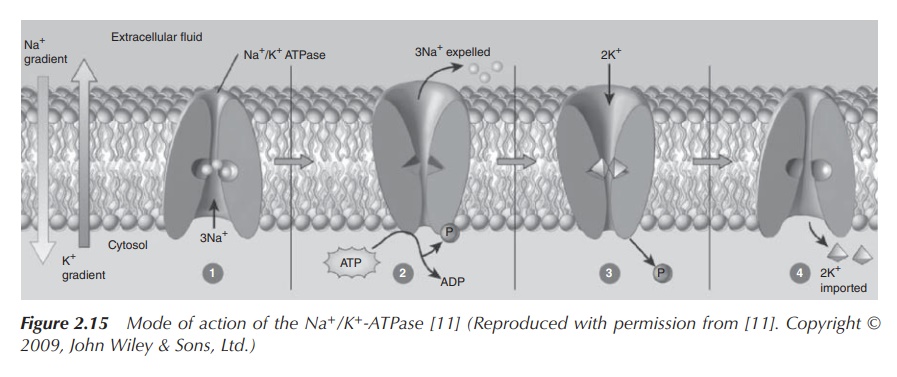
The Na+/K+ pumps facilitate an active
transport process which is based on the conformational changes of the
cross-membrane protein and driven by the breakdown of ATP. In the initial step,
three Na+ ions bind the cross-membrane protein on the cytosolic
side. This causes the protein to change its confirmation and makes it
accessible to ATP. In its new confirmation, the protein becomes phosphorylated
by ATP, which results in a second conformational change. The three Na+
ions are located across the membrane, and the protein now has a low affinity to
the sodium ions. This means that the sodium ions are dissociated from the
protein and released into the extracellular fluid. Nevertheless, the protein
has now a high affinity to K+ and binds two potassium ions from the
extracellular fluid. The bond phosphate is now dissociated, and the protein
reverts back to its original confirmation. This means both K+ ions are
exposed to the cytosol and can be released.
3. Drugs,
diet and toxicity
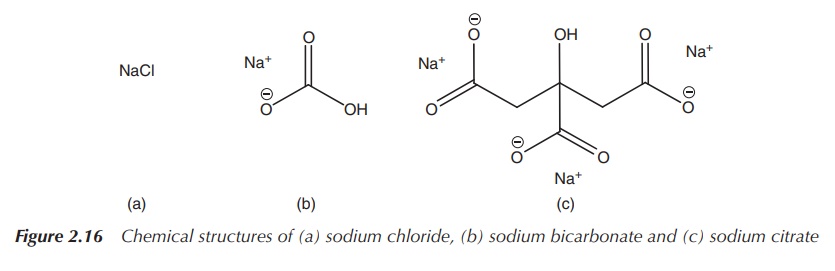
Sodium chloride solutions are normally used when the patient is
diagnosed with sodium depletion and dehy-dration. Treatment is mostly
administered intravenously, but in chronic conditions (mild to moderate sodium
loss) sodium chloride or sodium bicarbonate can be given orally. Oral
rehydration therapies usually use a mixture of alkali metal-based salts such as
NaCl, KCl and their citrates (Figure 2.16) .
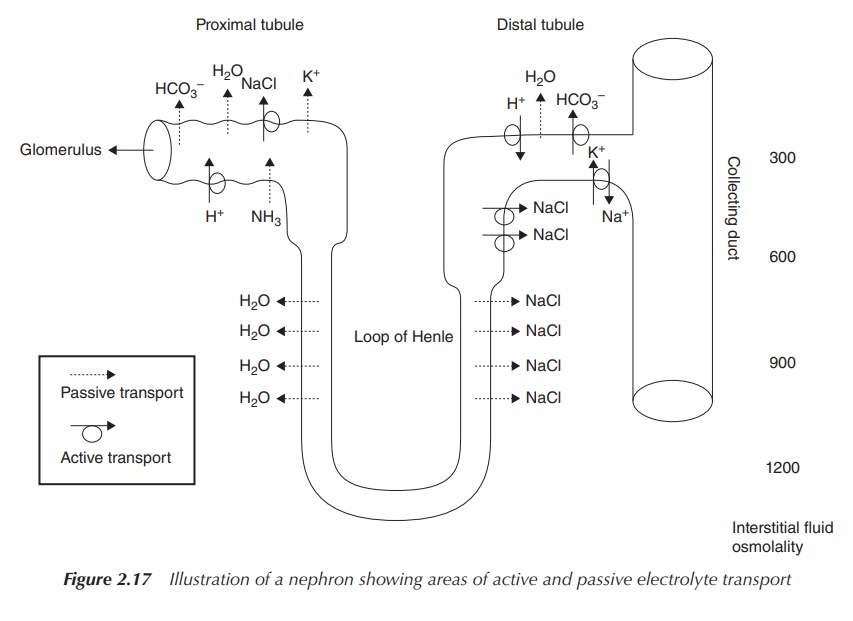
Sodium bicarbonate is usually administered orally in order to
regulate the serum pH. Imbalances of the plasma pH can be due to problems
occurring in the kidneys such as renal tubular acidosis. This is a medical
condition that occurs where the body accumulates acid as a result of the
kidneys failing to regulate the pH of the urine and the blood plasma. Within
the kidneys, blood is filtered before it passes through the tubular part of the
nephrons where re-absorption or secretion of important salts and others takes
place. In renal tubular acidosis, the kidneys either fail to filter or secrete
acid ions (H+) from the plasma (secretion takes place in the distal
tubule), or to recover bicarbonate ions (HCO3−) from the
filtrate (passive re-absorption takes place in the proximal tubule, active
re-absorption at the distal tubule), which is necessary to balance the pH. In
the view of this mode of action, the pharmaceutically active component of
sodium bicarbonate is the bicarbonate anion, but the cation Na+ is
responsible for solubility and compatibility (Figure 2.17) [3a].
The most common dietary source of NaCl is
table salt, which is used for seasoning and pickling (the high NaCl content
inhibits the bacterial and fungal growth as a result of the osmotic gradient).
The daily recommended NaCl intake varies depending on the country and the age
group. Within the United Kingdom,
Low sodium plasma levels (hyponatraemia), which again can be a
result of dysfunction kidneys or sodium loss in the bowels, also cause damage
to the human body via osmotic imbalances and if necessary have to be treated.
Low blood pressure, dehydration and muscle cramps are signs of a sodium
deficiency.
Signs of acute toxicity may be seen after ingestion of 500–1000 mg/kg body weight NaCl. These symptoms can be vomiting, ulceration of the gastrointestinal (GI) tract and renal damage. Also, the increased risk for the formation of kidney stones is believed to be a result of high salt intake .
Related Topics
