Sensitivity of Microorganisms
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Sterilization Procedures And Sterility Assurance
The general pattern of resistance of microorganisms to biocidal sterilization processes is independent of the type of agent employed (heat, radiation or gas), with vegetative forms of bacteria and fungi, along with the larger viruses, showing a greater sensitivity to sterilization processes than small viruses and bacterial or fungal spores.
SENSITIVITY OF MICROORGANISMS
The general pattern of resistance of
microorganisms to biocidal sterilization processes is independent of the type
of agent employed (heat, radiation or gas), with vegetative forms of bacteria
and fungi, along with the larger viruses, showing a greater sensitivity to
sterilization processes than small viruses and bacterial or fungal spores. The
choice of suitable reference organisms for testing the efficiency of
sterilization processes is therefore made from the most durable bacterial
spores; these are usually represented by Bacillus stearothermophilus for
moist heat, certain strains of B.subtilis for
dry heat and gaseous sterilization, and B.pumilus for
ionizing radiation.
Ideally, when considering the level of treatment necessary to achieve
sterility a knowledge of the type and total number of microorganisms present in
a product, together with their likely response to the proposed treatment, is
necessary. Without this information, however, it is usually assumed that
organisms within the load are no more resistant than the reference spores or
than specific resistant product isolates. In the latter case, it must be
remembered that resistance may be altered or lost entirely by repeated laboratory
subculture and the resistance characteristics of the maintained strain must be
regularly checked.
A sterilization process may thus be developed without a full microbiological
background to the product, instead being based on the ability to deal with a ‘worst
case’ condition. This is indeed the situation for official sterilization
methods, which must be capable of general application, and modern
pharmacopoeial recommendations are derived from a careful analysis of
experimental data on bacterial spore survival following treatments with heat,
ionizing radiation or gas.
However, the infectious agents responsible for spongiform
encephalopathies (prions) such as bovine spongiform encephalopathy (BSE) and
Creutzfeldt –Jakob disease (CJD) exhibit exceptional degrees of resistance to
many lethal agents. Recent work has even cast doubt on the adequacy of the
process of 18 minute exposure to steam at 134–138°C which has been recommended
for the destruction of prions (and which far exceeds the lethal treatment
required to achieve adequate destruction of bacterial spores).
a)
Survivor Curves
When exposed to a killing process,
populations of micro-organisms generally lose their viability in an exponential
fashion, independent of the initial number of organisms. This can be
represented graphically with a ‘survivor curve’ drawn from a plot of the
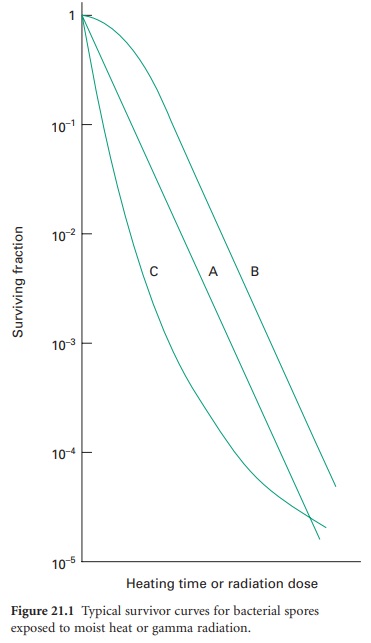
logarithm of the fraction of survivors against the exposure time or dose (Figure 21.1).
Of the typical curves obtained, all have a linear portion which may be
continuous (plot A), or may be modified by an initial shoulder (B) or by a
reduced rate of kill at low survivor levels (C). Furthermore, a short
activation phase, representing an initial increase in viable count, may be seen
during the heat treatment of certain bacterial spores. Survivor curves have
been employed principally in the examination of heat sterilization methods, but
can equally well be applied to any biocidal process.
b)
Expressions Of Resistance
i) D-value
The resistance of an organism to a
sterilizing agent can be described by means of the D-value. For heat and radiation treatments, respectively,
this is defined as the time taken at a fixed temperature or the radiation dose
required to achieve a 90% reduction in viable cells (i.e. a 1 log cycle
reduction in survivors; Figure 21.2A).
The calculation of the D-value assumes a
linear type A survivor curve (Figure 21.1),
and must be corrected to allow for any deviation from linearity with type B or
C curves. Some typical D-values for
resistant bacterial spores are given in Table 21.1.
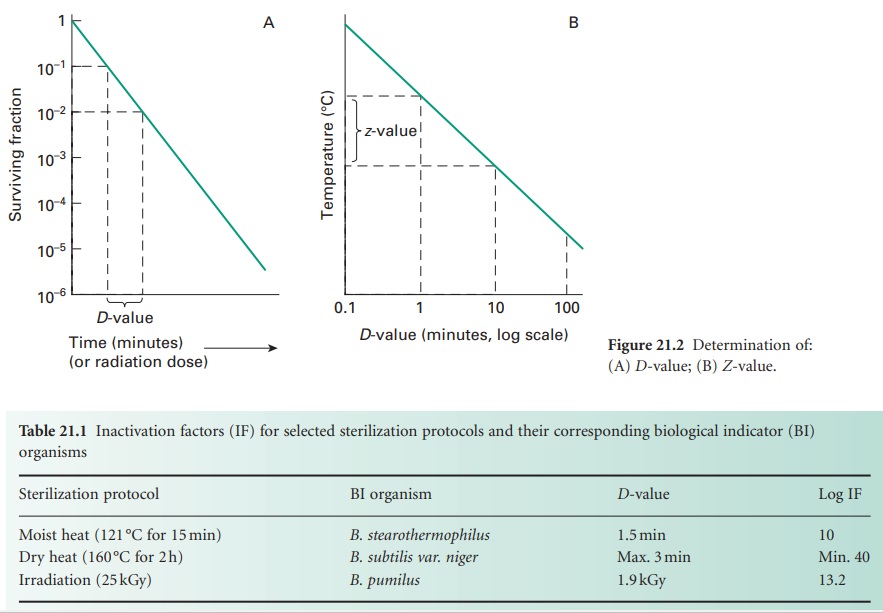
ii) Z-value
For heat treatment, a D-value only refers to the resistance of a
microorganism at a particular temperature. In order to assess the influence of
temperature changes on thermal resistance, a relationship between temperature
and log D-value can be developed, leading to the expression of
a Z-value, which represents the increase in temperature
needed to reduce the D-value of an
organism by 90% (i.e. 1 log cycle reduction; Figure 21.2 B).
For bacterial spores used as biological indicators for moist heat (B. stearothermophilus) and dry heat (B. subtilis) sterilization processes, mean Z-values are given as 10 °C and 22 °C, respectively.
The Z-value is not truly independent of temperature but may
be considered essentially constant over the temperature ranges used in heat
sterilization processes.
c)
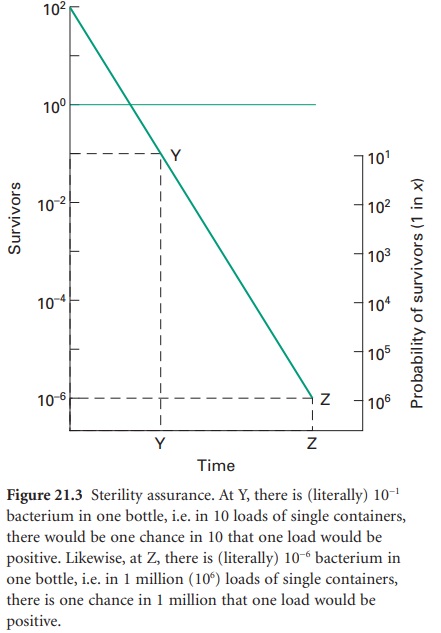
Sterility Assurance
The term ‘sterile’, in a microbiological context, means no surviving organisms whatsoever. Thus, there are no degrees of sterility; an item is either sterile or it is not, and so there are no levels of contamination which may be considered negligible or insignificant and therefore acceptable. From the survivor curves presented, it can be seen that the elimination of viable microorganisms from a product is a time-dependent process, and will be influenced by the rate and duration of biocidal action and the initial microbial contamination level. It is also evident from Figure 21.2 A that true sterility, represented by zero survivors, can only be achieved after an infinite exposure period or radiation dose. Clearly, then, it is illogical to claim, or expect, that a sterilization procedure will guarantee sterility. Thus, the likelihood of a product being produced free of microorganisms is best expressed in terms of the probability of an organism surviving the treatment process, a possibility not entertained in the absolute term ‘sterile’. From this approach has arisen the concept of sterility assurance or a microbial safety index which gives a numerical value to the probability of a single surviving organism remaining to contaminate a processed product. For pharmaceutical products, the most frequently applied standard is that the probability, post-sterilization, of a non-sterile unit is no more than 1 in 1 million units processed (i.e. ≤ 10− 6).The sterilization protocol necessary to achieve this with any given organism of known D-value can be established from the inactivation factor (IF) which may be defined as:
IF =10t̷D
where t is the contact time (for a heat or gaseous sterilization process) or dose (for ionizing radiation) and D is the D-value appropriate to the process employed.
Thus, for an initial burden of 102 spores an inactivation factor of 108 will be needed to give the required sterility
assurance of 10−6 (Figure 21.3).
The sterilization process will therefore need to produce sufficient lethality
to achieve an 8 log cycle reduction in viable organisms; this will require
exposure of the product to eight times the D-value of the
reference organism (8 D). In practice, it
is generally assumed that the contaminant will have the same resistance as the
relevant biological indicator spores unless full microbiological data are available
to indicate otherwise. The inactivation factors associated with certain
sterilization protocols and their biological indicator organisms are given
in Table 21.1.
Related Topics
