SAR of Tetracyclines
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antibiotics
The key structural feature is a linearly fused tetracyclic nucleus and each ring needs to be six membered and purely carbocyclic. A tetracyclic backbone skeleton is essential for activity.
SAR of Tetracyclines
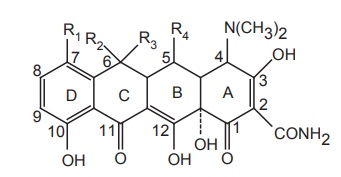
The key
structural feature is a linearly fused tetracyclic nucleus and each ring needs
to be six membered and purely carbocyclic. A tetracyclic backbone skeleton is
essential for activity.
·The D-ring needs to be aromatic and the A-ring
must be appropriately substituted at each of its carbon atoms for notable
activity.
·The B-ring and the C-ring tolerate certain
substitutent changes as long as the keto-enol systems (at C-11, 12, 12a) remain
intact and conjugated to the phenolic D-ring.
·The D, C, B-ring phenol, keto-enol system is
imperative and the A-ring must also contain a conjugated keto enol system.
·Specifically, the A-ring contains a tricarbonyl
derived keto-enol array at positions C-1, 2, and -3. Other structural
requirements for good antibacterial activity include a basic amine function at C-4
position of the A-ring.
Modification of C-1 and C-3 position: The keto-enol tautomerism of ring A in carbon
atom 1 and 3 is a common feature to all biologically active tetracyclines,
blocking this system by forming derivatives at C-1 and C-3 results in loss of
antibacterial activity A–C = O, a function of C-1 and C-3 is essential for
activity. In addition, equilibrium between non-ionized and Zwitterionic
structure of tetracycline is essential for activity.
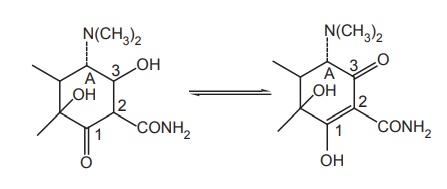
Modification of C-2 position: The antibacterial activity resides on the
carboxamide moiety. The amide is best left unsubstituted or monosubstitution is
acceptable in the form of activated alkylaminomethyl amide (Mannich bases). An
example includes rolitetracycline large alkyl group on the carboxamide that may
alter the normal keto-enol equilibrium of the C-1, 2, and 3 conjugated systems
and diminishes inherent antibacterial activity. The replacement of carboxamide
group or dehydration of carboxamide to the corresponding nitrile results in a
loss of activity.
Modification of C-4 position: The keto-enolic character of the A-ring is due
to the α-C-4 dimethyl amino substituent. Loss of activity is exerted when
dimethyl amino group is replaced with hydrazone oxime or hydroxyl group.
Modification of C-4a position: The α-hydrogen at C-4a position of tetracyclines
is necessary for useful antibacterial activity.
Modification of the C-5 and C-5a positions: Alkylation of the C-5 hydroxyl group results in
loss of activity. Naturally occurring antibacterial tetracyclines have an
unsubstituted methylene moiety at the C-5 position. However, oxytetracycline
contains C-5 α-hydroxyl group, was found to be a potent compound, and has been
modified chemically to some semisynthetic tetracyclines. Esterification is only
acceptable if the free oxytetracycline can be liberated in vivo; only small
alkyl esters are useful. Epimerization is detrimental to antibacterial
activity.
Modification at the C-6 position: The C-6 methyl group contributes little to the
activity of tetracycline. The C-6 position is tolerant to a variety of
substituents. The majority of tetracyclines have α-methyl group and α β-hydroxyl
group at this position. Demeclocyclin is a naturally occurring C-6 demethylated
chlortetracycline with an excellent activity. Removal of C-6 hydroxyl group
affords doxycycline, which exerts good antibacterial activity.
C-7 and C-9 substituents: The nature of the aromatic D-ring predisposes
the C-7 position to electrophilic substitution. Substitution with electron
withdrawing group such as nitro and halogen groups are introduced in some C-7
tetracyclines, which produces the most potent of all the tetracyclines in
vitro, but their are compounds are potentially toxic and carcinogenic. The C-7
acetoxy, azido, and hydroxyl tetracyclines are inferior in terms of
antibacterial activity.
C-10 substituents: The C-10 phenolic moiety is necessary for
antibacterial activity. C-10 substitution with para or ortho hydrogen group
activates the C-9 and C-7.
C-11 substituents: The C-11 carbonyl moiety is a part of one of the
conjugated keto-enol system required for antibacterial activity.
C-11a substituents: No stable tetracyclines are formed by
modifications at the C-11a position.
C-12/12a substituents: Esterification of the hydroxyl group leads to the
incorporation of drug with the tissues due to the enhanced lipophilicity and it
should undergo hydrolysis to leave the active tetracycline with hydroxyl group
at 12a position, which is necessary to produce good antibacterial action. The
transport and binding of these drugs depends on keto-enol system.
Related Topics
