SAR of Barbiturates
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Sedatives and Hypnotics
A good hypnotic barbituric acid derivative must have the following properties:
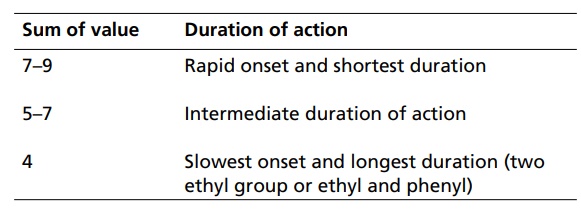
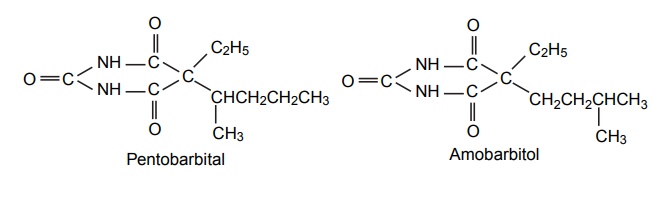
SAR of Barbiturates A good hypnotic barbituric acid derivative must have the following properties: The acidity value within certain limits to give proper ratio of ionized (dissociated) and unionized forms, which is important to cross blood brain barrier (BBB). It takes approximately 40%–60% dissociation to enable a barbiturate to cross BBB and exert effects on CNS. Determination of the pKa can thus be predictive of the CNS activity. Lipid water solubility (partition coefficient) should be in certain limits. 1. Acidity On the basis of acidity values, barbiturates are divided into two classes: Active class 5,5´-disubstituted barbituric acids 5,5´-disubstituted thiobarbituric acids 1,5,5´-trisubstituted barbituric acids Inactive class 1-substituted barbituric acids 5-substituted barbituric acids 1,3-disubstituted barbituric acids 1,5-disubstituted barbituric acids 1,3,5,5´-tetrasubstituted barbituric acids They are inactive since they are not acidic. These classes of agents depend on metabolism to produce 1,5,5´ trisubstituted barbituric acids, which are acidic. Attachment of alkyl substituent to both N1 and N3 renders the drug nonacidic, making them inactive. 2. Lipid water solubility Once the acidity value criteria is satisfied, the lipid-water solubility or partition co-efficient is calculated to find out whether the compound is active or not. The following structural skeleton is essential for hypnotic activity: The sum of the carbon atoms of both the substituents at c-5 should be between 6 and 10, in order to attain optimal hypnotic activity. This sum is also an index of the duration of action. Within the same series, the branched chain has greatest lipid solubility and hypnotic activity, but has shorter duration of action. Branched cyclic or unsaturated chains at C-5 position, generally, reduce the duration of action, due to increased ease of metabolic conversion to more polar inactive metabolite. The greater the branching, more potent will be the drug. Example: pentobarbital is more potent than amobarbital. However, the stero-isomers posses approximately the same potencies. Within the series, the unsaturated analogues (i.e. alkyl, alkenyl, and cycloalkenyl) may result in greater potency than the saturated analogues, with the same number of carbon atoms. Alicyclic or aromatic substituted analogues are more potent than analogues of aliphatic substitutions with the same number of carbon atoms. Introduction of a halogen atom into the C-5 substituents increase potency. Introduction of a polar substituents (OH, NH2, COOH, CO, RNH, and SO3H) into the aromatic group at C-5 results in decreased lipid solubility and potency. Alkylation at 1 or 3 position may result in compounds having shorter onset and duration of action since N-methyl group reduces acidity value. Replacement of oxygen by sulphur atoms at C-4 and C-6 position reduces the hypnotic activity. Replacement of oxygen by sulphur atom at C-2 position leads to rapid onset and shorter duration of action.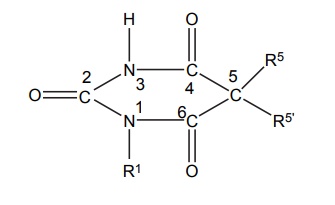
Related Topics
