Safety From Clinical Trial Data
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: The Efficacy and Safety of Selective Serotonin Reuptake Inhibitors for the Treatment of Depression in Children and Adolescents
Before the FDA Advisory Committee meeting of February 2, 2004, an anonymous press report stated that the planned presentation of the analysis of the safety of ATDs with respect to suicidality conducted by Andrew Mosholder was removed from the agenda.
SAFETY FROM CLINICAL TRIAL DATA
Before
the FDA Advisory Committee meeting of February 2, 2004, an anonymous press
report stated that the planned presentation of the analysis of the safety of
ATDs with respect to suicidality conducted by Andrew Mosholder was removed from
the agenda. Subsequently, a reclassification of the AEs reported in the trials
was conducted by Columbia University epidemiologists. After the revised data
were avail-able, the Mosholder analytic design [Office of Drug Safety (ODS)]
and a second analysis conducted by Turek Hammad for the Division of
Neuropharmaco-logical Drug Products (DNDP) were compared (Shen, 2003; Hammad,
2004; Mosholder, 2004). The analy-ses differ in that person-years was the unit
of analysis used in ODS and persons was the unit of analysis in DNDP. This
results in incident rate ratios for the former and relative risk estimates for
the latter. The studies also differ in the definition of suicidality AEs that
were captured in each study.
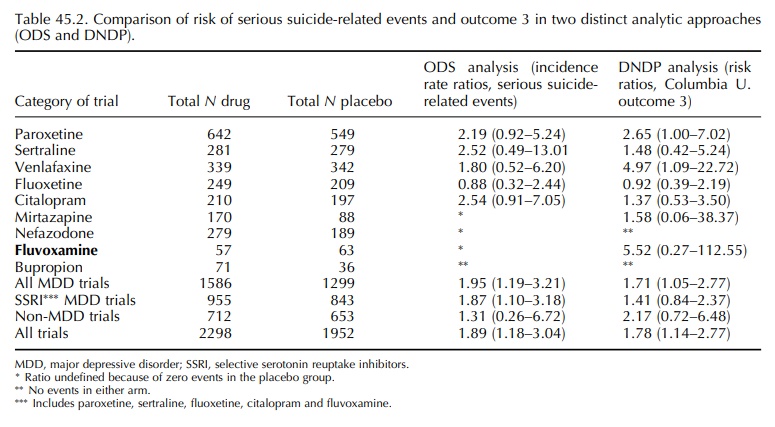
Table
45.2 depicts the results from 19 of the 23 trials evaluable for outcome 3
(suicide attempts and ideation) based on the Columbia revised data set (column
5) compared with the risk of serious suicide-related events according to the
standard regulatory definition: that is, life-threatening adverse drug
expe-rience, in-patient hospitalization or prolongation of hospitalization, or
disability/incapacity (column 4). There is little overall difference between
the two results for outcome 3 and serious suicide-related events. Both show an
increased risk for all MDD stud-ies. The total risk measure for the youth MDD trials
in which an SSRI was studied was 1.87 (1.10–3.18) in the ODS study and 1.41
(0.84–2.37) in the DNDP study. The analysis may be interpreted as show-ing a
weak ‘signal’ for risk of treatment-emergent suicidality although the DNDP
estimate includes 1 in the confidence interval – allowing the reviewer to
dismiss the importance of the signal. A possible reason for the variation
between the two results concerns whether serious suicide-related events and
outcome 3 (suicidal attempts and ideation) are comparable risks. In the case of
outcome 3, most events were ideation, an event that is likely to be three times
more preva-lent than attempts when lifetime self-reported data from adolescents
are examined (Evans et al., 2005) and
could account for reducing the risk estimate.
In
fact, measuring the risk for ideation alone = 78 compared with the risk for
suicidal behaviour = in
the risk estimates of suicidality was shown to dilute the risk [1.00
(0.52–1.94) vs. 1.83 (0.89–3.77)], respectively (Hammad, 2004, Table 5.10.36,
p. 38). It is noteworthy that SSRI use in MDD represents only 38% of the study
population in this analysis although this is the central question from a clinical
and consumer perspective. Equally important is the recog-nition that the
estimate for SSRI use in any individual trial would not achieve statistical
significance given the small sample sizes, brief duration, exclusion crite-ria
on suicide risk, volunteer bias and measurement inconsistencies (Avorn, 2005b).
In
the DNDP analysis, several potential effect modi-fiers were examined: a history
of suicidal behaviour, age and gender but none was different by treat-ment
group. An interesting sub-analysis conducted by Dr Hammad assessed
treatment-emergent hostil-ity or agitation. These symptoms may be reflec-tive
of the clinical condition referred to as activa-tion syndrome which has been
identified previously in SSRI studies and clinical practice (Wilens et al., 2003). It has been referred to
by various terms, for example akathisia (Lipinski et al., 1989), and is suspected of putting patients at greater risk
for suicidal behaviour or ideation (Teicher et
al., 1990; King et al., 1991).
Across all MDD trials, the risk of hostility and activation was significantly
elevated for SSRI-treated youths compared with placebo treated [2.34
(1.24–4.41)]. Overall, patients with symptoms of acti-vation or hostility were
up to 6.6 times more likely to have suicidality than those without such
activation (Hammad, 2004, slide 98). However, further analysis was not
undertaken because of the lack of informa-tion on the temporal pattern for
these symptoms with respect to reports of suicidality. Consequently, further
study of the relationship of treatment-emergent agita-tion, activation,
hostility and suicidal behaviours is likely to be more fruitful than these
initial broad anal-yses which, in the case of the DNDP analysis, focused on a
very broad operational definition of suicidality. In addition, age may be
crucial to further understanding AEs in relation to SSRI use. Using published
trials in which child and adolescent data were recorded sepa-rately, an
analysis of AEs, for example activation was two to three times more prevalent
in children than adolescents and accounted for more discontinuations than in
adults (Safer and Zito, 2006).
Treatment-emergent
events following ATD use have been studied using pharmacoepidemiologic data. An
analysis examining the association of ATDs with ‘treatment-emergent bipolar
disorder’ using a commercially-insured population aged 5–29 revealed that
children aged 10–14 years had the highest risk of ‘conversion to mania’ (Martin
et al., 2004). The term refers to the
sequential occurrence of a clinical diagnosis of mania following the use of an
ATD. The data rely on the validity of the physicians’ diagnoses and are subject
to alternative interpretation, that is the adverse symptoms may be indicative
of treatment-emergent activation rather than true mania.
Conclusions
from the ODS and DNDP analyses differed: Mosholder suggested the data from the
ODS study supported further analysis of events related to drug discontinuation
and proposed inpatient hospital-ization as an outcome. Hospitalizations might
shed light on the general problem of behavioural toxicities (new psychiatric or
behavioural symptoms following drug therapy for the control of psychiatric
symp-toms associated with medication for the treatment of psychiatric
symptoms). The sequence of these events is critical to infer causality – drug
exposure must precede new psychiatric symptoms. The history of past events is
also critical. Loss of symptoms upon discontinuation of the drug (dechallenge)
would offer supportive evidence of an association. By contrast, the DNDP
analysis reviewer concluded that ‘the strength of the suicidality signal,
although it varies from drug to drug, is comparable to previous findings for
most drugs’, a statement that seems to nullify the signal.
A
number of limitations of these FDA-sponsored analyses should be considered:
First,
searching clinical trial data restricts the assess-ment to a very small
drug-exposed population. In this case, there were approximately 2000 youths
with major depressive disorder, mainly adolescents who were exposed to an SSRI.
Since suicide events in a lifetime estimate for adolescents were esti-mated at
10%, it would appear that in a 4–6 week trial the likelihood of this occurring
is slight if not totally improbable, because the study is not powered to find such
rare events. Second, trial participants are subject to volunteer bias.
Exclusion of suicidal patients was likely to increase selection bias which
makes the assessment of suicidality from clinical trial data particularly
troubling. The positive gain from having youths randomized to drug and placebo
condi-tions to avoid channelling or other treatment bias found in
community-treated populations is offset by the selection biases produced by the
use of trial data (e.g. exclusion of suicidal behaviour and volun-teer bias).
Third, measurement bias may further limit the analysis, because the
overwhelming proportion of suicidal events in the DNDP outcome 3-analysis
relied on suicidal ideation reports which resulted in a weak non-significant
risk estimate. The prediction of completed suicide from suicide attempts for
15–19 year old boys is 400:1 and 3000:1 for girls, whereas for suicidal
ideation it is 9000:1 for boys and 19 000:1 for girls. Such ratios in children
and adolescents are lower than that for adults (Mann, 2006) and render the FDA
safety analyses from clinical trial data insuf-ficient. Fourth, the short
duration of the trials may miss the window when risk is greatest if it occurs
after 4–6 weeks, the typical length of the trials in the study. Consequently,
it is useful to review the safety findings from observational studies. These
limi-tations, notwithstanding, leading psychopharmacol-ogy researchers have
concluded that the signal from the trials is not sufficient to support a risk
of suici-dality (Mann, 2006). It is also instructive that the conclusions from
these analyses did not urge funding for priority research initiatives to
address the safety question in a more precise epidemiological fashion (Avorn,
2005b).
Related Topics
