Retrosynthetic Analysis
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Planning Organic Syntheses
The last several chapters have been concerned with learning how to manipulate functional groups, how to make carbon–carbon bonds, and ways to put pieces of molecules together.
Planning Organic Syntheses
RETROSYNTHETIC ANALYSIS
The
last several chapters have been concerned with learning how to manipulate
functional groups, how to make carbon–carbon bonds, and ways to put pieces of
molecules together. Next all of these ideas must be integrated into the general
idea of synthetic planning. If we are given a particular molecule to synthesize
(target), we must be able to plan the actual chemical route to be used in the
preparation of the target. The task is to devise a strategy whereby a
particular starting material is converted by a series of steps (reactions) to
the desired target.
One
fact must be recognized at the outset—that the target is the compound which
must be produced from the starting material. This rather obvious statement of
the problem is often overlooked by students, but it lies at the heart of
synthetic planning. Targets are chosen in order to achieve some purpose. A
particular target might be chosen as a drug candidate, or as a potential
insecticide, or as a motor oil additive. Whatever its purpose, a target will
have particular structural features that must be produced by the synthetic
sequence. Getting close won’t do!
Starting
materials can be chosen by a variety of criteria. For example, a par-ticular
starting material might have the same carbon skeleton as the target, it might
be a by-product of a chemical plant and therefore be readily available, it
might be very cheap and easily obtained from a specialty chemical company, or
it might contain a chiral center necessary in the target. Whatever the reason,
a particular starting material imposes certain requirements on the synthetic
route that must be taken to produce the target.
To
transform the starting material into the target, reactions must be chosen which
will accomplish the conversion efficiently and with the correct selectiv-ity.
Reactions which do not or cannot deliver the target are without merit for the
synthesis of that particular target. This relatively simple idea is also often
forgotten, and it is common to force a particular reaction to give a product
even though the oxidation level, reactivity, regioselectivity, and/or
stereochemistry of the reaction are wrong for the target being considered.
Herein
lies one of the difficulties of synthetic planning. We tend to learn organic
reactions in the forward direction—that is, reactants A and B give product C.
This type of information handling is a convergent process in that a set of
conditions is imposed which leads to a limited number of potential outcomes—in
most cases a single product!
A
+ B → C
Yet
synthetic planning asks the opposite question—what reactants are nec-essary to
give product C? This requires that we think backward from products to
reactants. This type of problem solving is a divergent process in that a great
many potential reactants are possible and many possibilities must be explored
before any one set of reactants is chosen as the solution.
Let’s
look at a very simple one-step synthesis to illustrate this difference. Given
the following set of reactants, methyl cyclohexanol and chromic acid in
acetone, it is very easy to write the product as 4-methylcyclohexanone:
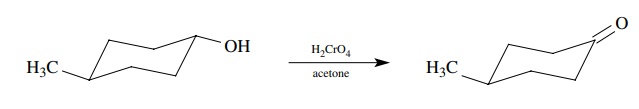
This
is because we have learned that secondary alcohols are oxidized effi-ciently to
ketones by chromic acid and recognize this as a Jones oxidation. If
4-methylcyclohexanol is recognized as a secondary alcohol, then it must give a
ketone with Jones reagent.
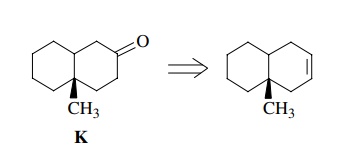
In
contrast, if we are asked to synthesize the target bicyclic ketone K shown below, we must work backward.
If we had an alcohol A of the same
carbon skeleton, then we could oxidize it to the ketone by a Jones oxidation.
This step is indicated by a double arrow (⇒) and is called a
retrosynthetic step. It shows how we work backward (“retro”) from the target K to a given starting material A. (Of course, the actual synthesis
would be carried out in the opposite direction—from the starting material A to the product K.) Alternatively oxidative cleavage (O3) of olefin O would furnish the target, as would
the periodate cleavage of exocyclic diol x-D.
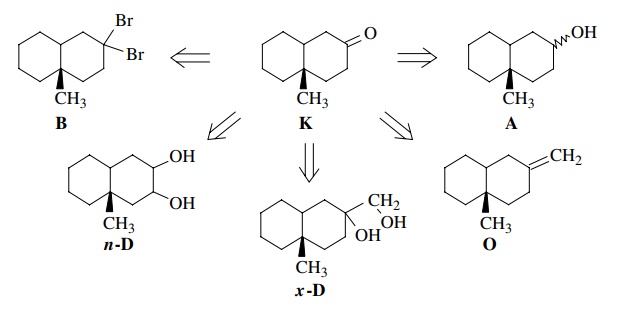
Pinacol
rearrangement of endocyclic diol n-D and hydrolysis of dibromide B would also furnish the target K. Thus each retrosynthetic step in our
backward analysis corresponds to a synthetic step to the target in the forward
direction.
Although
it is possible to generate a variety of potential solutions to the synthetic
task at hand, the problem is not yet solved. The validity of each step must be
checked. For each retrosynthetic step to be valid, there must be a reaction or
reagent capable of effecting the transformation with needed chemospecificity,
regiospecificity, and stereospecificity to give the target compound as the
major product. In the example written above, oxidation of an alcohol (A) to a ketone (K) is a very facile step, and there are many ways to do this
conversion so it is a valid retrosynthetic step. Likewise the olefin cleavage (O) or the diol cleavage (x-D)
are also well known to be clean and would yield the target. Conversely the
pinacol rearrangement of n-D would probably not be
regiospecific, and the hydrolysis of dibromide B is a very messy reaction which often gives complex mixtures
rather than single products. These latter two retrosynthetic solutions are
therefore invalid solutions to the synthetic problem.
Moreover
the following retrosynthetic analysis for the preparation of K is not valid either because there is
no good reaction to directly convert an olefin to a ketone:
The
olefin could be converted to an alcohol and then the alcohol could be oxidized
to the target ketone, but this is actually two sequential reactions, not one.
They must be written as
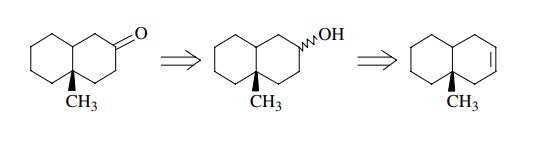
Here
we see that the first retrosynthetic step, the preparation of a ketone from an
alcohol, is valid as discussed above; however, the second retrosynthetic step
is not. Although the addition of water across a double bond is a
straightforward, common reaction, its use in the present example requires that
hydration take place regiospecifically even though there is no significant
control element present to ensure the needed regioselectivity. Thus a mixture
of alcohols is expected from the hydration reaction rather than the
single-alcohol regioisomer needed for oxidation to the target ketone.
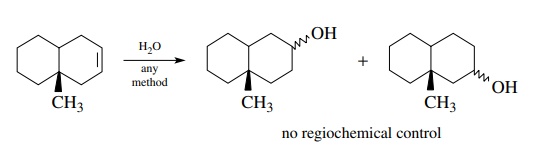
For
each retrosynthetic step to be a valid solution, the reactants must give the
appropriate product with needed structural features and control in the for-ward
direction, that is, the direction taken in the actual synthesis. Thus each
retrosynthetic step must be checked both forward and backward to effectively
plan workable synthetic routes to new molecules.
To
carry out an effective retrosynthetic analysis, one must keep in mind certain
basic features which must be dealt with during the synthesis. These include
functional groups, oxidation levels, and stereochemistry. In addition, it is
often necessary to construct the carbon skeleton so carbon–carbon bond-forming
processes must be integrated into the sequence at the best juncture all the
while keeping in mind the above considerations.
Related Topics
