Prenatal Development
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Pregnancy and Development
Prenatal Development : Cleavage: Blastocyst Formation, Implantation, Placentation: Embryonic Development, Gastrulation, Development of the Fetal Circulation
Prenatal
Development
The time spent in prenatal development is called gestation . The gestation
period occurs
from the last menstrual
period until birth, which takes approx-imately 280 days. A gestation time of
less than 37 weeks is referred to as “premature.” If gestation continues beyond
42 weeks, it is considered “post-mature,” regardless of the size of the fetus or other factors. A developing offspring is
referred to with a variety of names based on the period of development. The
fertilized egg is called a conceptus
or, more com-monly, a zygote. It undergoes mitosis about 30 hours after it has
formed. The period from fertilization through the Eighth week of gestation is
known as the embryonic
period, with the zygote now being called an embryo. The period from the Ninth week of gestation through birth
is known as the fetal
period, with the embryo now being called a fetus. At birth, the fetus is then called an infant. During the first period of gestation, all major organ
systems appear.
Cleavage
Cleavage
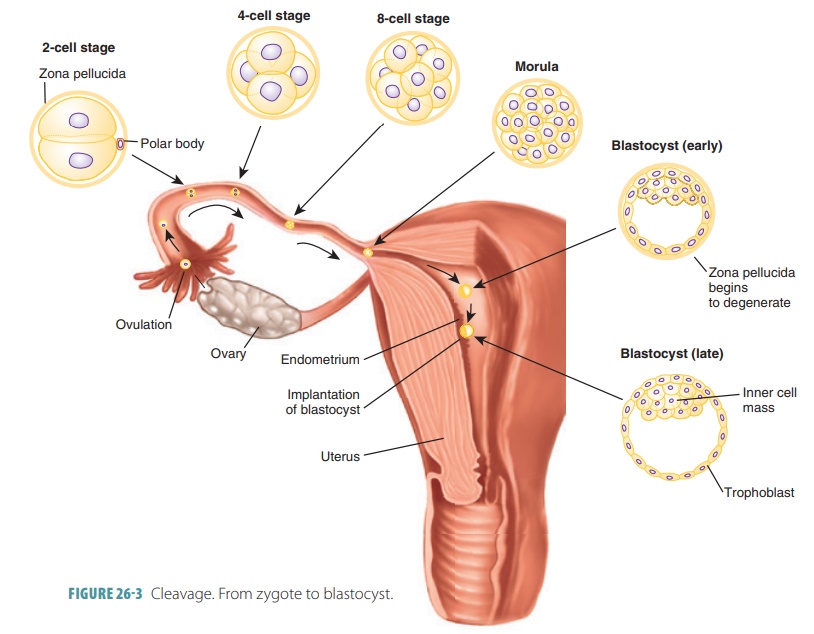
is the series of repeated mitotic cell divi-sions that occur in an
ovum immediately after fertil-ization (FIGURE 26-3). During this period, the tiny cell mass moves
through the uterine tube to the uterine cavity. This takes about three days,
with the structure consisting of a solid “ball” of 16 or more cells that is
called a morula.
The tiny dividing cells have a high surface-to-volume ratio, allowing them to
uptake oxygen and nutrients while disposing of wastes. The quickly dividing
cells begin to build embryonic structures.
Blastocyst Formation
From the initial single fertilized egg cell, there is
divi-sion into two blastomeres. These,
in turn, mitoti-cally divide into four cells, then eight, and so on. By the
Fifth day after fertilization, the embryo is made up of approximately 100
cells. It has begun accumulating fluid within an internal cavity and floats
freely inside the uterus. During this time, the zona pellucida degen-erates.
The morula becomes a hollow blastocyst, which is
basically a ball of cells that attaches to the endometrium. The blastocyst is
filled with fluid and made up of one layer of large flat trophoblast cells as well as an inner cell mass of
20–30 rounded, clustered cells. Therefore, the sequence of preembryonic
struc-tures is as follows: zygote, morula, and blastocyst.
Soon, the trophoblast cells develop L-selectin adhe-sion
surface molecules. They aid in the formation of the placenta and secrete
several immunosuppressive fac-tors, which protect the trophoblast and embryo
from being attacked by the mother’s cells. The embryo forms from the inner cell
mass after it forms the embryonic disc. Three extraembryonic membranes also form from the embryonic disc. The fourth membrane,
called the chorion, is formed from
the trophoblast.
Implantation
After about one week, the developing offspring has become
superficially implanted in the endome-trium (FIGURE 26-4). Until this point, the cells that will form the developing
offspring, known as plu-ripotent stem cells, can give rise to specialized
cells, including additional stem cells. Uterine secretions, rich in
glycoproteins, steroids, vitamins, and other nutrients, nourish the
blastocyst. The window of implantation describes how receptive
the endo-metrium is to implantation. This occurs because of surging ovarian
hormone levels—estrogens and progesterone—in theblood.
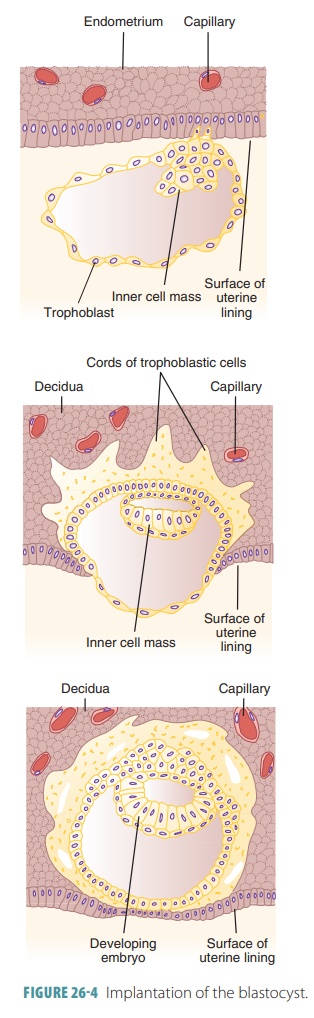
When the endometrium is receptive, integrin and selectin
proteins located on the trophoblast cells bind to extracellular matrix
components and selectin- binding carbohydrates, respectively, located on the
inner uterine wall. The extracellular matrix compo-nents are collagen,
fibronectin, laminin, and other components. The blastocyst is implanted in the
upper area of the uterus. Shortly after implantation, the tro-phoblast forms
two distinct layers.
The placenta produces several hormones during pregnancy. Human chorionic gonadotropin (hCG),
estrogen, and progesterone are the main hormones. hCG is secreted by
trophoblast cells and maintains via-bility of the corpus luteum. It bypasses
the effects of the hypothalamus, pituitary, and ovaries, prompting the corpus
luteum to continually secrete progesterone and estrogen. This is controlled by
the chorion. Therefore, the
developing offspring assumes hormonal control of the uterus in this period. hCG
is not only similar to luteinizing
hormone in its effects, but also has prote-ase activity, promoting
development of the placenta by acting as an autocrine growth factor. In areas
where the trophoblast “faces” the endometrium, hCG levels are higher. The
placenta also releases human placental
lactogen, which is also called human chorionic soma-tomammotropin.
This polypeptide hormone functions similarly
to growth hormone. It modifies the mother’s metabolism to facilitate the energy
supply of the fetus and also has anti-insulin properties.
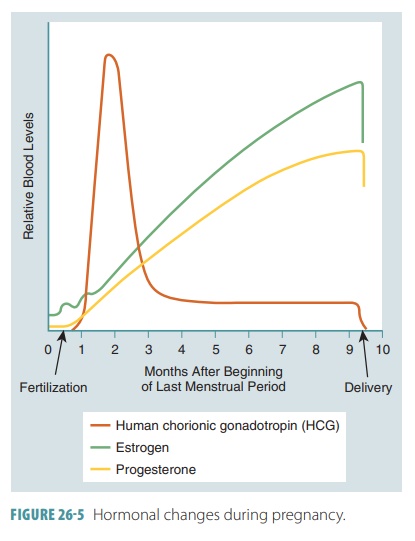
Levels of hCG are often detectable in the mother’s blood
just one week after fertilization. They rise until the end of the second month
of development. Levels then decline sharply, reaching their lowest point at
four months of gestation, and remain low from this point on (FIGURE 26-5). All pregnancy tests are actually
antibody tests, detecting hCG in a mother’s blood or urine. At the Eighth week
of development, the basic structural form of the human body is recognizable and
the embryo is renamed a fetus. Simple versions of all organs are present.
These organs and other structures enlarge and become specialized as the fetus
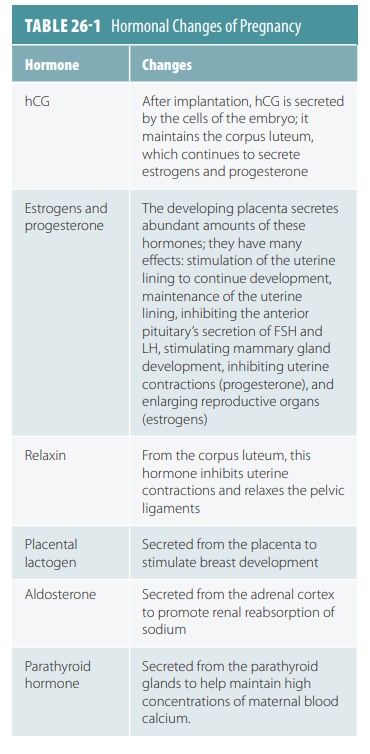
develops. The hormonal changes of pregnancy are summarized in TABLE 26-1.
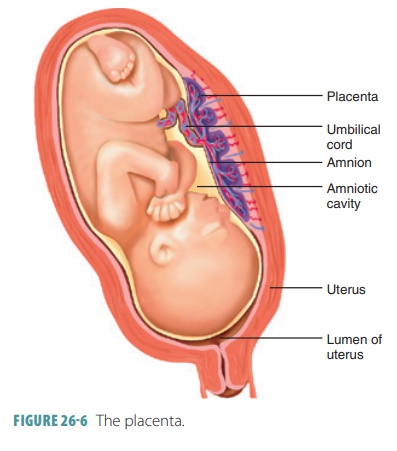
Placentation
Placentation
is the formation of the
placenta (FIGURE
26- 6). This temporary structure takes over production
of progesterone and estrogen produc-tion for the remainder of the pregnancy,
beginning in between the second and third months of gestation. The corpus
luteum has degenerated by this time and the ovaries are inactive until birth
occurs.
A layer of extraembryonic mesoderm develops from cells
derived from the original inner cell mass. They line the inner surface of the
trophoblast, forming the chorion, which
develops chorionic villi. These
finger-like structures are more elaborate in structure where they contact the
maternal blood. Blood ves-sels form in their cores, extending to the embryo to
form the umbilical arteries and vein. It is important to understand that
originally all the blastocyst is sur-rounded by the chorionic villi. As the
chorion enlarges, it expands like a balloon inside the endometrium In week
four, the embryo, amnion, and yolk sac are suspended inside a growing,
fluid-filled chamber.
Blood-filled lacunae are
formed in the stratum functionalis of the endometrium. The villi are
contin-ually nourished by extravascularized maternal blood. During
placentation, the distal parts of the allantois and blood vessels carrying
blood in and out of the pla-centa are contained in the body stalk, which connects the embryo and chorion. The yolk stalk is a narrow structure that
connects the endoderm of the embryo with the yolk sac.
Beneath the embryo, the present endometrium becomes the decidua basalis, whereas the endome-trium
surrounding the uterine cavity in the area of implantation becomes the decidua capsularis. The
decidua basalis is located between the developing embryo and the myometrium.
The placenta is actually formed by the combination of the decidua basalis and
the chorionic villi. The decidua capsularis expands to accommodate the fetus,
filling and stretching the uter-ine cavity over time. The villi in the decidua
capsu-laris become compressed and degenerate, and the villi in the decidua
basalis proliferate and become more branched. The remainder of the uterine
endometrium, which has no contact with the chorion, is called the decidua parietialis.
By the third month of gestation, the placenta is usually
fully functional. It supplies nutrition, oxygen, hormones, and also removes
wastes. Barriers exist that prevent free passage of substances between the two
blood supplies: the chorionic villi membranes and the embryonic capillaries of
the endothelium.
Normally, the maternal and embryonic blood sup-plies do not
mix.
Blood levels of estrogens and progesterone contin-ually
increase throughout pregnancy. The uterine wall is maintained during the second
and third trimesters by placental estrogens and placental progesterone. The
placenta also secretes the hormone known as placen-tal lactogen, which helps to
stimulate breast devel-opment and prepares the mammary glands for milk
secretion. The relaxin
hormone is also produced by the placenta. After birth, the placenta detaches
and sloughs off.
1. What is cleavage?
2. Differentiate an embryo from a fetus.
3. What is a morula?
4. Which hormone suppresses uterine contractions until the
birth process begins?
5. What are
lacunae?
Embryonic Development
The process of embryonic development during and after
implantation involves many significant steps. The blastocyst is converted to a gastrula and the three primary germ layers form. The extraembryonic membranes develop.
Before developing three layers, the
inner cell mass divides into two layers known as the upper epiblast and the lower hypoblast.
The inner cell mass is then called the embryonic
disc. The extra-embryonic membranes form during weeks two and three of
development and are the amnion,
yolk sac,
allantois,
and chorion.
The amnion is a transparent, membranous sac that develops
from cells of the epiblast and fills with amniotic fluid. As the embryonic disc
eventually curves
and forms the tubular body, the amnion also curves. The sac eventually extends
entirely around the embryo, with its only break being the umbilical cord. The amnion protects the
embryo against trauma and maintains a constant temperature. The amniotic fluid
prevents the growing structures of the embryo from sticking together or fusing,
while allowing freedom of movement. The amniotic fluid is first formed from
maternal blood, but the growing functionality of the embryo’s kidneys means
that fetal urine later contrib-utes to the amniotic fluid.
From cells of the primitive gut, the yolk sac forms, which
hangs from the embryo’s ventral surface. With the embryonic disc being the
point of contact, the amnion and yolk sac appear like two balloons that touch
each other. Human eggs contain only small amounts of yolk, with nutritive
functions being assumed by the placenta. The yolk sac is important for two main
reasons: it forms part of the embryo’s diges-tive tube and it is the source of
the first blood cells and vessels that form.
The allantois forms at the caudal end of the yolk sac as a
small formation of embryonic tissue. It is the structural basis for the
umbilical cord, which links the embryo to the placenta. The allantois
eventually becomes part of the urinary bladder. When fully formed, the
umbilical cord has a core of embryonic connective tissue that is also called Wharton’s jelly.
The core also contains the umbilical arteries and vein and
is covered by amniotic membrane externally. As described earlier, the chorion
helps to form the pla-centa. It is the outermost membrane, enclosing the
embryonic body and each of the other membranes.
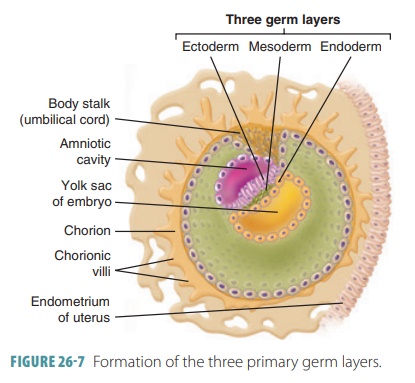
Gastrulation
The processes of gastrulation begin with
a primitive streak appearing on the
embryonic disc’s dorsal
surface. On day 12 of embryonic develop-ment, a new layer forms through
gastrulation. This groove with raised edges creates the longitudinal axis of
the embryo. Epiblast cells on the surface of the embryonic disc move medially
over other cells to enter the primitive streak. The first cells enter-ing the
groove displace the yolk sac’s hypoblast cells to form the inferior germ layer
known as the endoderm
(FIGURE 26-7).
The cells that follow move laterally between the cells at the upper and lower
surfaces to form the mesoderm
. Mesodermal cells just beneath aggregate to form a rod of cells known as the notochord. This is the first axial
support of the embryo. Cells that remain on the dorsal surface of the embryo
make up the ectoderm. The embryo is now about
2 mm long.
Organogenesis
In organogenesis, the
primary germ layers of the embryo begin to develop the organs. Organogene-sis
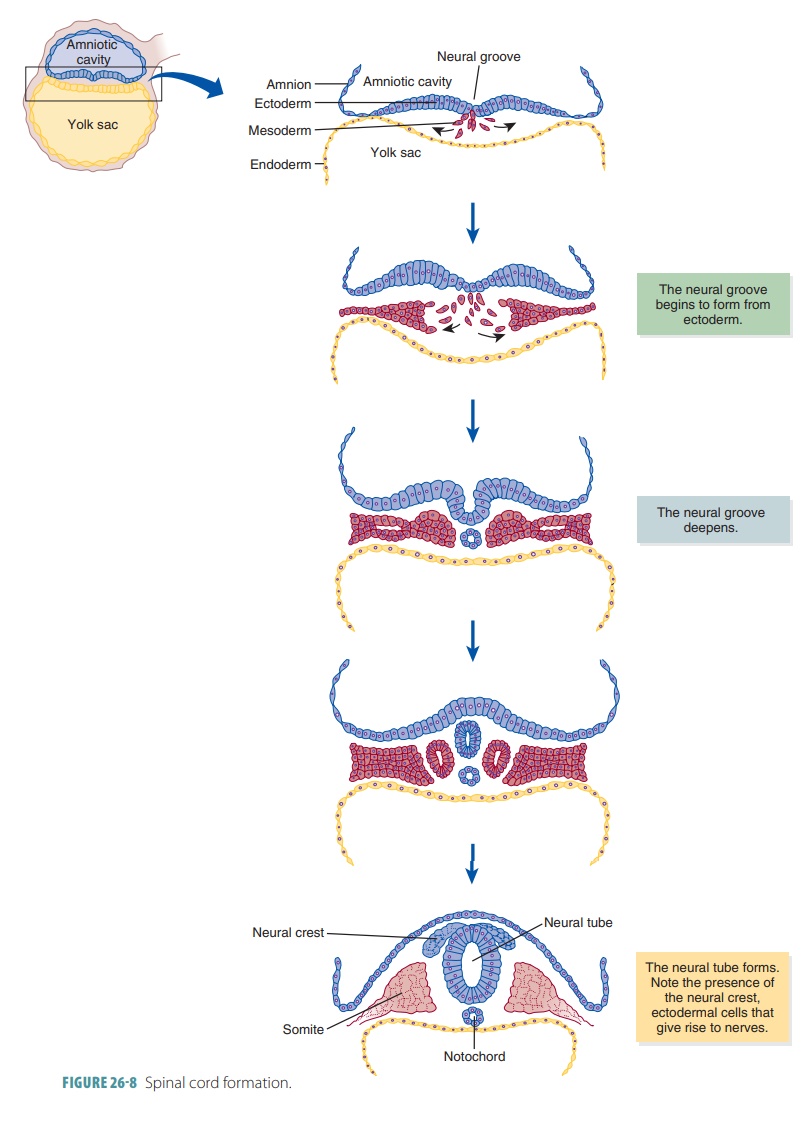
is the primary event of embryonic development (TABLE 26 -2). The formation of the spinal cord and brain are among the
first events of organogenesis. These structures rise from the ectoderm of the
inner cell mass. FIGURE
26 -8 shows how the spinal cord forms during this period. The
ectoderm folds inward, creat-ing the neural groove along the embryo’s entire
pos-terior surface. The neural groove deepens and then closes over in a few
weeks, creating the neural
tube. This structure’s walls thicken to form the spinal cord and
expand to form the brain. The spinal and cranial nerves develop from the
ectodermal cells that form the neural
crest. These cells form axons that attach to other organs of the
body as well as the bones, muscles, and skin.
The mesoderm forms deeper muscles, bones, car-tilages, and
other structures. Much of it first forms the somites, which then form the backbone and the head and trunk
muscles. Mesoderm that is lateral to the somites becomes the dermis of the
skin, the con-nective tissues, and the limb bones and muscles. The endoderm of
the inner cell mass forms the yolk sac, with the upper part of the yolk sac
forming the intes-tinal tract lining. The yolk sac also forms the blood cells
and primitive germ cells. The germ cells move from the yolk sac wall to the
developing ovaries and testes. These cells become the oogonia in females and
the spermatogonia in males.
Cellular Formation of the Fetus
The following cells produce different parts of the
developing fetus:
■■ Ectodermal
cells: Nervous system, parts of special sensory organs, epidermis, hair,
nails, skin glands,linings of mouth and anal canal
■■ Mesodermal
cells: Muscle tissues, bone tissue,bone marrow, blood, blood vessels,
lymphatic vessels, connective tissue, internal reproductive organs, kidneys,
epithelial linings of body cavities
■■ Endodermal
cells: Digestive tract epithelium, respiratory tract, urinary bladder,
urethra.
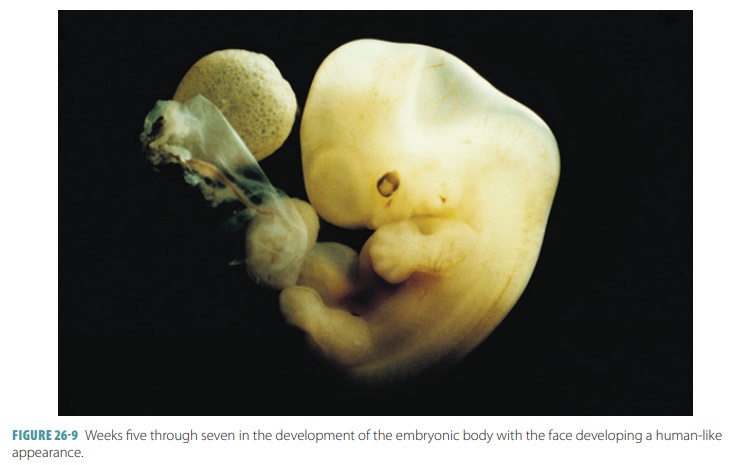
The flat embryonic disc becomes cylindrical, with the head
and jaws developing by the end of the fourth week. The heart is now beating,
forcing blood through the blood vessels, and tiny buds form, which will become
the upper and lower limbs.
The head grows quickly and becomes rounded and erect between
the fifth and seventh weeks, with the facial features developing. The limbs
elongate and the fingers and toes appear. Programmed cell death or apoptosis forms them from the
preexisting “webbing.” At the end of the seventh week, all of the most
critical internal organs are present.
Past the eighth week, only the villi that remain in contact
with the endometrium endure. The others degenerate, with their former locations
smoothing. The part of the chorion still contacting the uterine wall becomes
the placenta. A thin placental
membrane separates embryonic blood inside the capillary of a
chorionic villus from the maternal blood in a lacuna. Maternal and embryonic
blood exchange substances across this membrane (FIGURE 26-9). Oxygen and nutrients diffuse from the maternal blood into
the embryo’s blood. Carbon dioxide and other wastes dif-fuse from the embryo’s
blood into the maternal blood. Using active transport and pinocytosis, various
sub-stances also cross the placental membrane.
1. Describe how the placenta forms.
2. Explain the various developmental milestones of the embryo.
3. How are substances exchanged between the embryo’s blood and
the maternal blood?
4. What is a gastrula?
5. Define
the terms “umbilical cord” and “yolk sac.”
Development of the Fetal Circulation
Maternal blood supplies oxygen and nutrients while car-rying
away wastes, diffusing these substances through the placental membrane. The
first blood cells develop in the yolk sac. Before week three, spaces appear in
the splanchnic mesoderm that are soon lined by endothe-lial cells and covered
with mesenchyme. They are linked together with quickly growing vascular
networks that will form the heart, blood vessels, and lymphatics.
By the end of the third week, the embryo has a paired blood
vessel system. The two vessels that form the heart have fused and are not bent
to form an “S” shape. Just 3–4 days later, the heart is already pump-ing blood,
although the embryo is less than one-fourth inch in length. The umbilical arteries
and vein as well as three vascular shunts form. They are all occluded when
birth eventually occurs. The umbilical vein car-ries freshly oxygenated blood
from the placenta into the embryo’s body, bringing it to the developing liver.
After birth, the umbilical vein becomes the ligamentum teres.
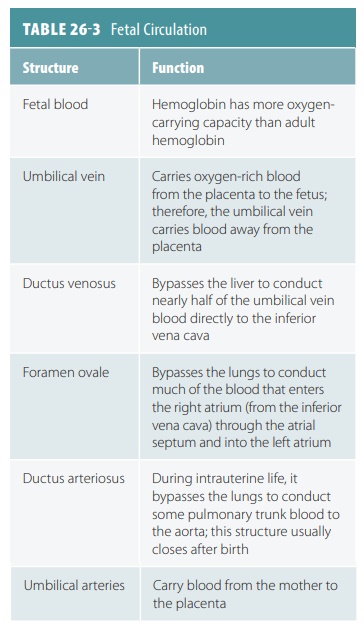
Fetal blood contains about 50% more oxygen- carrying hemoglobin than maternal blood. Fetal hemoglobin can carry up to 30% more oxygen than adult hemoglobin. The path of blood in the fetal cardio-vascular system is shown in FIGURE 26-10.
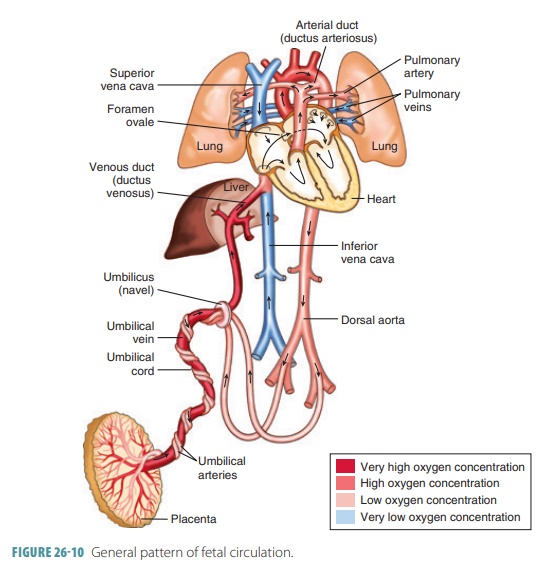
Nearly half the blood carried to the fetus via the umbilical vein passes into the liver, with the rest enter-ing the ductus venosus, which bypasses the liver. This vessel extends to join the inferior vena cava, where oxygenated blood from the placenta mixes with deoxygenated blood from the lower areas of the fetal body. This blood then continues on to the right atrium. Because fetal lungs are nonfunctional, blood largely bypasses them. Much of the blood entering the fetal right atrium is moved directly into the left atrium through an opening in the atrial septum called the foramen ovale. Blood pressure is slightly greater in the right atrium than the left atrium. A small valve helps prevent blood flow from reversing. In the liver, blood flow is important to ensure the health of the liver cells. After birth, the infant’s liver assumes the functions of nutrient processing that are handled by the mother’s liver during gestation.
The rest of the right atrium blood passes into the right
ventricle and out through the pulmonary trunk.The pulmonary blood vessels have
a high resistance to blood flow during this period of development, but enough
blood reaches them to sustain them. Most of the pulmonary trunk blood enters a
fetal vessel called the ductus
arteriosus, connecting to the descending portion of the aortic arch.
Blood low in oxygen is pre-vented from entering the portion of the aorta
branch-ing to the heart and brain.
A mixture of highly oxygenated blood entering the left
atrium and a small amount of deoxygenated blood from the pulmonary veins moves
into the left ventricle and is pumped into the aorta; some reach the myocardium
and some reach the brain. Blood from the descending aorta moves to the lower
regions of the body, with the rest passing into the umbilical arteries leading
to the placenta. There, it is reoxygen-ated. TABLE 26-3 summarizes fetal circulation. At birth, the fetal
cardiovascular system must adjust when the placenta stops functioning and the
newborn begins to breathe.
Growth During the Fetal Period
Teratogens
are environmental factors that cause con-genital malformations by
interfering with prenatal growth or development. Among the known teratogens are
chemical agents, including drugs such as thalido-mide and alcohol; infectious
agents, especially German measles; and ionizing radiation (x-rays). The fetal period begins at the end of the eighth week and lasts until birth. Growth occurs rapidly
during this period, with body proportions beginning to appear more like those
of a normal infant. Growth of the head begins to slow as growth of the body
increases. By the 12th week, the external reproductive organs may be
distinguished as either male or female. FIGURE 26-11 shows fetal devel-opment at 5–6 weeks, four
months, and five months.
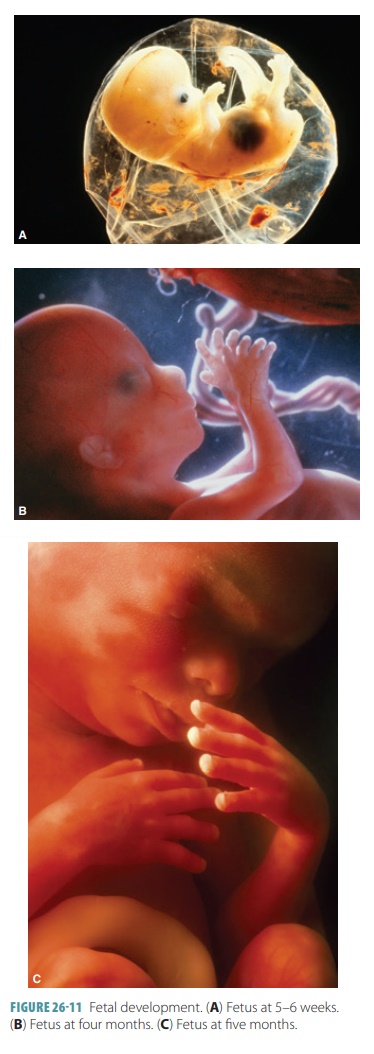
The fetus grows rapidly during the fourth month, reaching up
to 20 cm in length as the limbs lengthen and the skeleton continues to ossify.
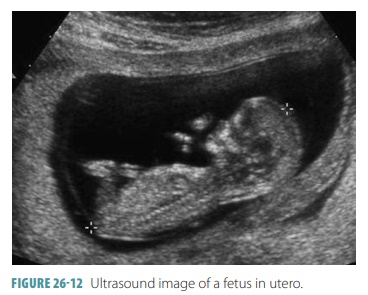
Ultrasound may be used to assess a fetus in utero during pregnancy (FIGURE 26-12). Tests show that four months old
fetuses turn away from bright lights if flashed on the moth-er’s belly and show
reactions to loud noises. Growth slows during the fifth month and the lower
limbs have reached their final relative proportions. The mother may feel
movement beginning around this time. The fetus begins to grow hair on its head,
and the skin of the body is covered in fine hair and a mixture of dead
epidermal cells and sebum from the sebaceous glands.
During the sixth month, the fetus gains substan-tial weight
and the eyebrows and eyelashes grow. The skin is wrinkled, translucent, and
reddish in appearance because of the many blood vessels. In the seventh month,
fat is deposited in subcutaneous tissues, smoothing the skin. The eyelids,
which were fused during the third month, reopen. By the end of the seventh
month, the fetus is about 40 cm long.
During the final trimester, brain cells form net-works,
organs specialize and grow, and fat continues to develop beneath the skin. The
testes of the male fetus descend into the scrotum. The final systems to mature
are the digestive and respiratory systems; hence, many babies have difficulty
breathing and digesting milk from the mother. At the end of the ninth month or,
more accurately, after 266 days, the fetus is considered full term. By now, it
is nearly 50 cm long and weighs between 2.7 and 3.6 kg. The skin has lost its
fine hair but is still coated with sebum and dead epidermal cells. The scalp is
usually covered with hair. Nails have developed on the fingers and toes and the
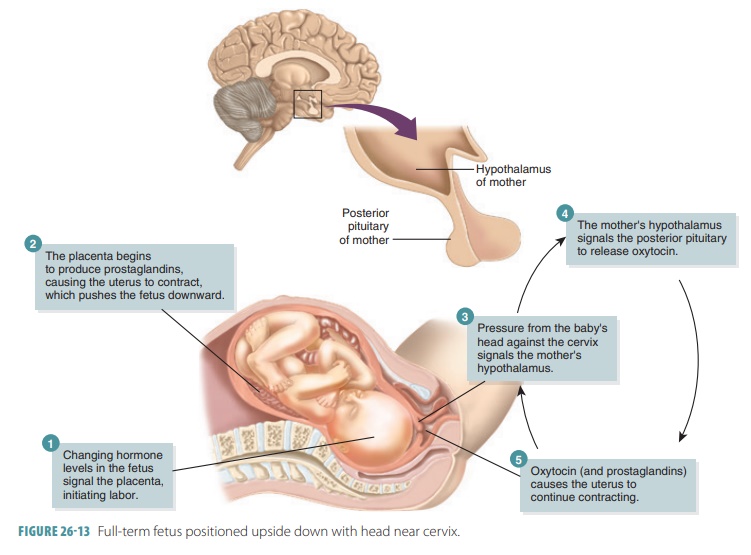
skull bones are largely ossified. The fetus is normally positioned upside down
with the head toward the mother’s cervix (FIGURE 26-13). During pregnancy, the respiratory rate and tidal vol-ume
increase. Maternal blood volume, the glomerular filtration rate, and nutrient
requirements also increase.