Platinum anticancer agents
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Transition Metals and d-Block Meta Chemistry
Despite the success of cisplatin, there is a need to develop new platinum anticancer drugs.
Platinum
anticancer agents
Despite the success of cisplatin, there is a need to develop new platinum anticancer drugs. Cisplatin is a very toxic compound and can have severe side effects such as nephrotoxicity (kidney poisoning), ototoxicity (loss of high-frequency hearing) and peripheral neuropathy (damage to nerves of the peripheral nervous system), although it is possible to control some of these effects. Very typically, cancer cells can become resistant to cisplatin after repeated administration.
This is a fairly common
problem experienced at the repeat treatment with cisplatin. Furthermore,
compounds active against a variety of cancer types are required to combat
cancer.
Two second-generation platinum drugs are so far successfully
registered worldwide – carboplatin and oxaliplatin. There are others such as
nedaplatin, which is registered in Japan for the treatment of head and neck,
testicular, lung, cervical, ovarian and nonsmall-cell lung cancer. In South
Korea, heptaplatin is used against gastric cancer, whereas lobaplatin is
licensed in China for the treatment of cancers including metastatic breast
cancer, small-cell lung cancer and myelogenous leukaemia .
Nevertheless, the development of new platinum-based drugs has
been less successful than expected. The majority of compounds are not used in a
clinical setting because their efficacy is too low, toxicity is too high or the
compounds showed a poor aqueous solubility, a fairly common problem for
transition-metal-based compounds.
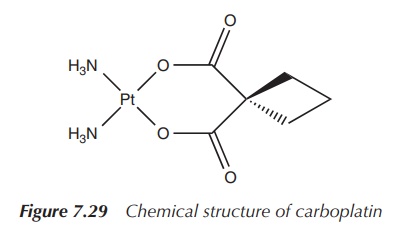
1. Carboplatin
Carboplatin, cis-diammine(1,1-cyclobutanedicarboxylato)platinum(II), is a
second-generation platinum drug. Its structure is based on cisplatin with the
difference that the chloride ligands are exchanged for a bidentate chelating
ligand. A consequence is that carboplatin is less reactive than cisplatin and
therefore is less nephro-toxic and orthotoxic than the parent compound.
Unfortunately, it is more myelosuppressive than cisplatin, which reduces the
patients’ white blood cell count and makes them susceptible to infections .
Carboplatin was licensed by the FDA in 1989 under the brand name Paraplatin and
has since then gained worldwide recognition. Carboplatin on its own or in
combination with other anticancer agents is used in the treatment of a variety
of cancer types including head and neck, ovarian, small-cell lung, testicular
cancer and others (Figure 7.29).
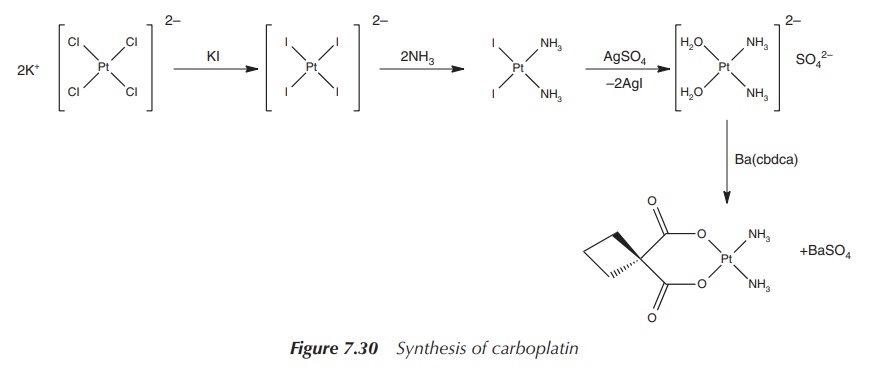
Carboplatin is a pale-white solid showing good aqueous
solubility. The synthesis starts with potassium tetrachloroplatinate, which is
reacted to the orange [PtI4]2− anion. Analogous to the
synthesis of cisplatin, in the following steps the anion is reacted with
ammonia (due to the translabellizing effect of iodide, the ammonia ligands are
directed into the cis position) and converted to cis-[Pt(NH3)(H2O)2]SO4.
In the final step, the complex is reacted with the chelating agent Ba(cbda)
(cbdca, cyclobutane-1,1-dicarboxylate) and carboplatin is formed (Figure 7.30).
Carboplatin is administered by intravenous (IV) injection. A
typical solution contains the drug in a high concentration in water or in a
mannitol or dextrose solution. The dose is determined either on the basis of
the body surface area of the patient or according to their renal function. The
doses are typically three to six times higher than cisplatin, which reflects
the lower chemical reactivity and toxicity of this drug. Carboplatin can be
given on an outpatient basis, as it is better tolerated than cisplatin.
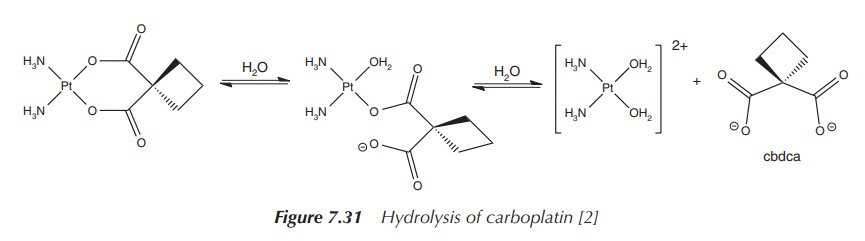
The mode of action relies on the ring-opening of the cbdca
chelate ring and interaction of the platinum centre with DNA. Carboplatin is
seen as a prodrug, which itself is not very reactive within the human body but
once activated shows its full potential. Hydrolysis of carboplatin and removal
of the chelate ligand or at least the opening of the ring makes this compound
much more cytotoxic than the parent compound itself (Figure 7.31).
In vitro studies have shown that the drug binds to DNA and forms initially a mono-functional adduct, which over time is converted into the di-functional platinum–DNA adduct. There are indications that carboplatin forms DNA intrastrand cross-links analogous to cisplatin, but its reactivity towards DNA is reduced .
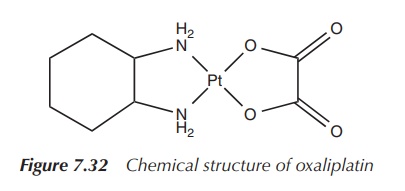
2. Oxaliplatin
Oxaliplatin (cis-[oxalato]
trans-1,2-diaminocyclohexane platinum(II)),
for example, marketed under the trade name Eloxatin, is considered as a
third-generation platinum-based anticancer drug. Its structure differs from
previously synthesised platinum compounds by the configuration of its amino
substituents. Its platinum centre is coordinated by two chelating ligands,
namely an oxalate ligand and a so-called DACH (1,2-diaminocyclohexane) ligand.
In comparison to cisplatin, the two chlorine leaving groups are replaced by an
oxalato leaving group. The simple amino groups are replaced by the DACH ligand,
which is the nonleaving group (Figure 7.32).
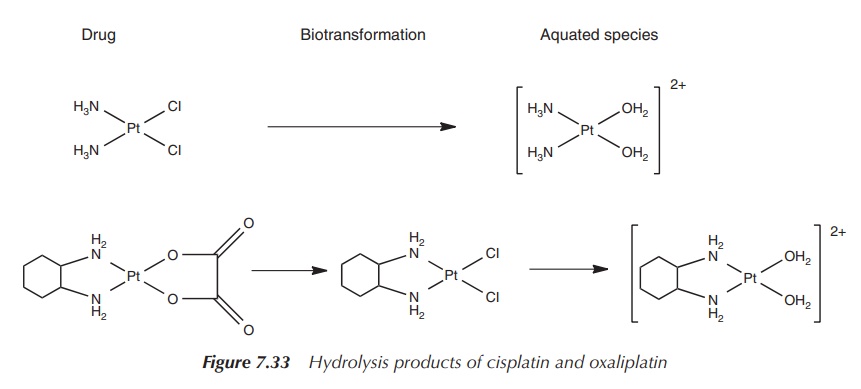
Cisplatin and carboplatin are hydrolysed to a common
diamino-platinum species, whereas the hydrolysis product of oxaliplatin
contains the bulky DACH group, which sterically hinders the DNA repair
mechanism. These mismatch repair enzymes are particularly active in colon
cancer and, not surprisingly, oxaliplatin shows excellent activity in the
treatment of colon and rectal cancers
(Figure 7.33).
The clinical use of oxaliplatin was approved by the European
Union in 1999 and by the FDA in 2002. It is most effective in combination with
5-fluorouracil and leucovorin (5-FU/LV) in the treatment of metastatic
carcinomas of the colon or rectum . Oxaliplatin induces less side effects than
cisplatin; for example, it is less nephrotoxic and ototoxic and leads to less
myelosuppression. Unfortunately, treatment with oxaliplatin can lead to nerve
damage, which may not be reversible in the case of chronic exposure of the
patient to the drug. Oxaliplatin is usually administered intravenously as
infusion over a period of 2–6 h in doses similar to cisplatin. The neurotoxic
side effects are dose-limiting .
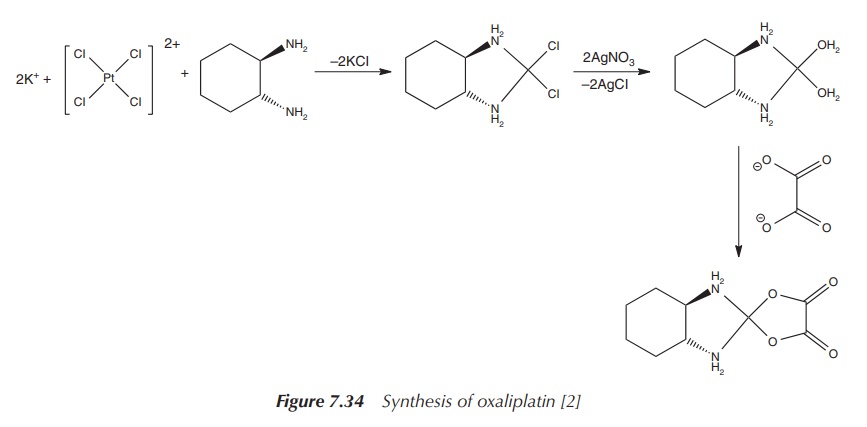
The synthesis of oxaliplatin starts with K2[PtCl4], the same starting material used for the synthesis of carboplatin. This is reacted with water and 1 equiv of the nonleaving ligand, 1R,2R-DACH ligand.
Note that there are different stereoisomers of the DACH ligand, and
cytotoxicity studies have shown that the use of this specific stereoisomer 1R,2R-DACH
leads to the most potent compound. Upon treatment with silver nitrate, the
diaqua complex is formed. Any excess of silver ions can be removed by adding
potassium iodide. This leads to the formation of the insoluble silver iodide,
which can be filtered off. The diaquo platinum complex is subsequently treated
with 1 equiv of oxalic acid, and oxaliplatin is formed as a solid (Figure
7.34).
It is believed that DNA is the major cellular target of
oxaliplatin, as researchers have shown that it forms intrastrand cross-links
similar to cisplatin. The oxaliplatin–DNA adduct also leads to a bending of the
DNA similar to the cisplatin–DNA adduct. Nevertheless, there are significant
differences to the cisplatin–DNA adduct. The oxaliplatin–DNA adduct forces a
narrow minor groove bend (helix bend of 31∘), whereas the equivalent cisplatin–DNA adduct
leads to a wide minor groove (60–80∘). Also, it has been observed in the solid
state structure of the oxaliplatin–DNA adduct that there is a hydrogen bond
formed between the NH of the DACH group and the oxygen atom of guanine base,
which interacts with the platinum centre .
3. Other platinum drug candidates
There are numerous platinum compounds under research for their
potential use as anticancer agents. Only a few of them have found their way
into the clinic so far, with cisplatin, carboplatin and oxaliplatin being the
most successful ones.
One example is nedaplatin, cis-diammineglycolatoplatinum(II),
which is structurally similar to carboplatin. The chemical structure consists
of a central platinum(II) atom with two cis-ammonia
groups as nonleaving groups and – in contrast to carboplatin – the dianionic
form of glycolic acid as the leaving group. Nedaplatin has been approved for
the clinical use in the Japanese market for the treatment of head and neck,
testicular, ovarian, lung and cervical cancer. It is typically administered by
IV injection and its dose-limiting side effect is myelosuppression (Figure
7.35).
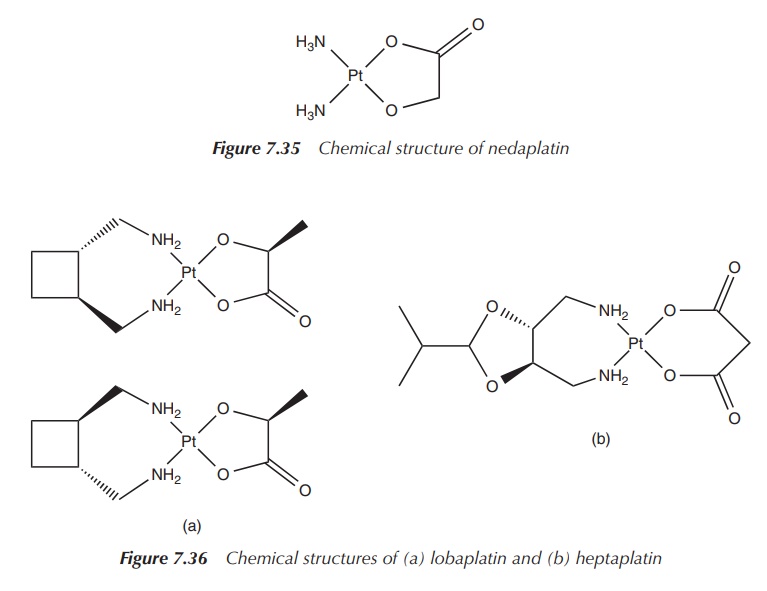
Lobaplatin and heptaplatin are further examples of platinum-based agents being used in China and South Korea, respectively. Lobaplatin is used in the treatment of nonsmall-cell lung cancer and breast cancer. Hep-taplatin is used in South Korea to treat gastric cancer. Both drugs show the typical side effects such as myelosuppression and mild hepatotoxicity. Their success is limited and has not led to approval in the EU or by the FDA (Figure 7.36).
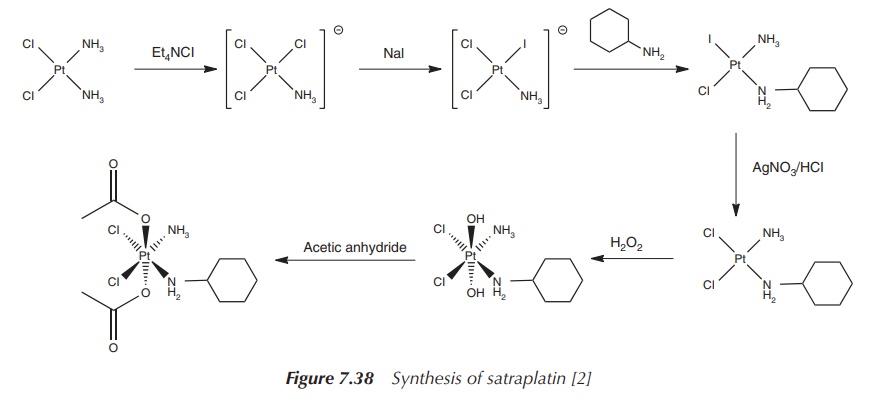
Satraplatin (JM216, cis,trans,cis-[PtCl2(OAc)2(NH3)(C6H5NH2)])
is a Pt(IV) or Pt4+ complex, which is active by oral administration,
as it is more hydrophobic than cisplatin. This form of administration is very
attractive because of the convenience and freedom it provides to the patient.
Satraplatin also has a milder tox-icity profile and is shows no
cross-resistance with cisplatin. Satraplatin in combination with prednisone has
completed phase III clinical trials against hormone-refractory prostate cancer.
The results were very encour-aging, but the overall survival rate did not
improve significantly enough. As a result, the fast-track approval of the FDA
was not granted (Figure 7.37).
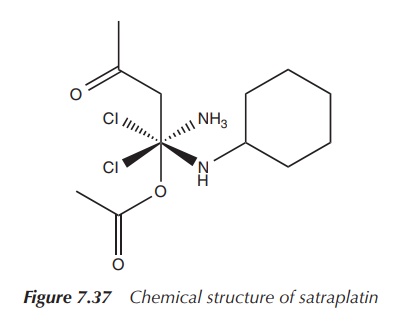
Structurally, satraplatin consists of a Pt(IV) centre, which is
coordinated by six ligands forming a close to octahedral geometry. In general,
octahedral Pt(IV) complexes (low-spin d6) are much more kinetically
inert than square planar Pt(II) complexes. Pt(IV) complexes can be readily
reduced in vivo to Pt(II) by
reductants such as ascorbate or thiols (e.g. cysteine, GSH).
The synthesis of satraplatin starts with cisplatin, which is
reacted with tetraethylammonium chloride (Et4NCl) – a source of Cl−.
As a result of the trans-directing effect, the iodide ligand is introduced in a
second step adjacent to the ammonia group. Subsequently, 1 equiv of
cyclohexylamine is added, which coordinates to the platinum centre trans to the
iodide. Silver nitrate is used to remove the iodide ligand, as no further
‘trans-directing’ action is required. The Pt2+ is finally oxidised
to Pt4+, which expands the coordination sphere from 4 to 6 –
octahedral geometry. In the last step, the acetate ligands are introduced
(Figure 7.38).
Satraplatin is the only orally administered platinum-based drug
that has entered clinical trials so far. The difficulty for this administration
route lies in the aggressive conditions that are present in the stomach. In
general, metal complexes do not survive the acidic conditions in the stomach
and therefore will not reach the gastrointestinal (GI) tract unchanged. The
advantage of satraplatin is that the complex is relatively inert to any
exchange reactions and therefore has an increased chance of reaching the
pH-neutral GI tract unchanged. From here, the drug enters the blood stream. In vitro studies with fresh human blood
have shown that within minutes the reduction of the platinum centre to Pt2+
takes place in the red blood cells. This may be facilitated by haemoglobin,
cytochrome c and NADH, and leads to a square planar Pt2+ complex
containing the chloride and ammonia ligands .
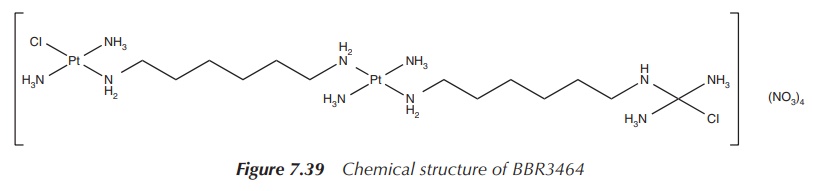
Further research has led to the development of multi-platinum
complexes. This is against the ‘rules’ set out for platinum-based anticancer
drugs, which state that a successful drug candidate should consist of only one
platinum centre with amine-based nonleaving groups in cis position and two
leaving groups, also in cis position. Clearly, polynuclear platinum compounds
fall outside these rules, but researchers have synthesised the unusual
trinuclear complex BBR3464, which was very successful in in vitro studies and even reached clinical trials against melanoma
and metastatic lung and pancreatic cancer
(Figure 7.39).
Related Topics
