Nucleotide Structure
| Home | | Biochemistry |Chapter: Biochemistry : Nucleotide Metabolism
Nucleotides are composed of a nitrogenous base; a pentose monosaccharide; and one, two, or three phosphate groups. The nitrogen-containing bases belong to two families of compounds: the purines and the pyrimidines.
NUCLEOTIDE STRUCTURE
Nucleotides are
composed of a nitrogenous base; a pentose monosaccharide; and one, two, or
three phosphate groups. The nitrogen-containing bases belong to two families of
compounds: the purines and the pyrimidines.
A. Purine and pyrimidine structures
Both DNA and RNA
contain the same purine bases: adenine (A) and guanine (G). Both DNA and RNA
contain the pyrimidine cytosine (C), but they differ in their second pyrimidine
base: DNA contains thymine (T), whereas RNA contains uracil (U). T and U differ
in that only T has a methyl group (Figure 22.1). Unusual (modified) bases are
occasionally found in some species of DNA and RNA (for example, in some viral
DNA) and in transfer RNA (tRNA). Base modifications include methylation,
glycosylation, acetylation, and reduction. Some examples of unusual bases are
shown in Figure 22.2. [Note: The presence of an unusual base in a nucleotide
sequence may aid in its recognition by specific enzymes or protect it from
being degraded by nucleases.]
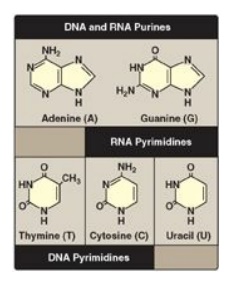
Figure 22.1 Purines and pyrimidines commonly found in DNA and RNA.
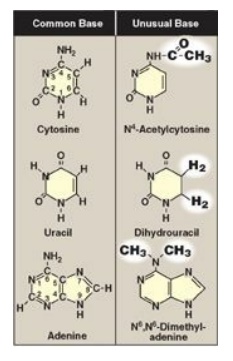
Figure 22.2 Examples of
unusual bases.
B. Nucleosides
The addition of a
pentose sugar to a base through a glycosidic bond produces a nucleoside. If the
sugar is ribose, a ribonucleoside is produced, and if the sugar is 2-deoxyribose,
a deoxyribonucleoside is produced (Figure 22.3A). The ribonucleosides of A, G,
C, and U are named adenosine, guanosine, cytidine, and uridine, respectively.
The deoxyribonucleosides of A, G, C, and T have the added prefix, “deoxy-” (for
example, deoxyadenosine). [Note: The compound deoxythymidine is often simply
called thymidine, with the “deoxy-” prefix being understood, because it is
incorporated into DNA only.] The carbon and nitrogen atoms in the rings of the
base and the sugar are numbered separately (Figure 22.3B). Note that the
carbons in the pentose are numbered 1 to 5. Thus, when the 5-carbon of a
nucleoside (or nucleotide) is referred to, a carbon atom in the pentose, rather
than an atom in the base, is being specified.
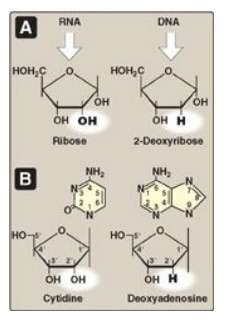
Figure 22.3 A. Pentoses found in nucleic acids. B. Examples of the numbering systems for purine- and pyrimidinecontaining nucleosides.
C. Nucleotides
The addition of one or
more phosphate groups to a nucleoside produces a nucleotide. The first
phosphate group is attached by an ester linkage to the 5 I -OH of the pentose, forming a nucleoside 5I -phosphate or a 5 I -nucleotide. The type of pentose is
denoted by the prefix in the names “5 I -ribonucleotide” and “5 I -deoxyribonucleotide.” If one phosphate group is attached to the
5 I -carbon of the pentose, the
structure is a nucleoside monophosphate, like adenosine monophosphate [AMP]
also called adenylate). If a second or third phosphate is added to the
nucleoside, a nucleoside diphosphate (for example, adenosine diphosphate [ADP]
or triphosphate (for example, adenosine triphosphate [ATP]) results (Figure
22.4). The second and third phosphates are each connected to the nucleotide by
a “high-energy” bond. [Note: The phosphate groups are responsible for the
negative charges associated with nucleotides and cause DNA and RNA to be
referred to as “nucleic acids.”]
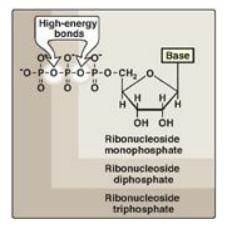
Figure 22.4 Ribonucleoside
monophosphate, diphosphate, and triphosphate.
Related Topics
