Michaelis-Menten Kinetics
| Home | | Biochemistry |Chapter: Biochemistry : Enzymes
Leonor Michaelis and Maude Menten proposed a simple model that accounts for most of the features of enzyme-catalyzed reactions.
MICHAELIS-MENTEN KINETICS
A. Reaction model
Leonor Michaelis and
Maude Menten proposed a simple model that accounts for most of the features of
enzyme-catalyzed reactions. In this model, the enzyme reversibly combines with
its substrate to form an ES complex that subsequently yields product,
regenerating the free enzyme. The model, involving one substrate molecule, is
represented below:
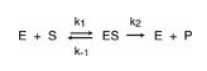
where
S is the substrate
E is the enzyme
ES is the
enzyme–substrate complex
P is the product
k1, k-1,
and k2 are rate constants
B. Michaelis-Menten equation
The Michaelis-Menten
equation describes how reaction velocity varies with substrate concentration:

where
Vo = initial reaction
velocity
Vmax = maximal velocity
Km = Michaelis constant
= (k-1 + k2)/k1
[S] = substrate
concentration
The following
assumptions are made in deriving the Michaelis-Menten rate equation:
1. Relative concentrations of enzyme and substrate: The concentration of substrate
([S]) is much greater than the concentration of enzyme ([E]), so that the
percentage of total substrate bound by the enzyme at any one time is small.
2. Steady-state assumption: [ES] does not change with time (the steady-state assumption), that is, the rate of formation of ES is equal to that of the breakdown of ES (to E + S and to E + P). In general, an intermediate in a series of reactions is said to be in steady state when its rate of synthesis is equal to its rate of degradation.
3. Initial velocity: Initial reaction velocities (vo) are used in the analysis of enzyme reactions. This means that the rate of the reaction is measured as soon as enzyme and substrate are mixed. At that time, the concentration of product is very small, and, therefore, the rate of the back reaction from product to substrate can be ignored.
C. Important conclusions
1. Characteristics of Km: Km, the Michaelis constant, is
characteristic of an enzyme and its particular substrate and reflects the
affinity of the enzyme for that substrate. Km is numerically equal to the
substrate concentration at which the reaction velocity is equal to 1⁄2 Vmax. Km
does not vary with enzyme concentration.
a. Small Km: A numerically small (low) Km reflects a
high affinity of the enzyme for substrate, because a low concentration of
substrate is needed to half-saturate the enzyme—that is, to reach a velocity
that is 1⁄2 Vmax (Figure 5.9).
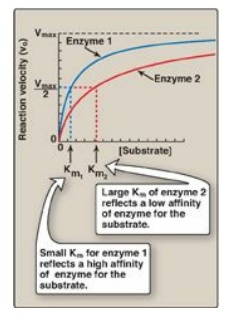
Figure 5.9 Effect of substrate concentration on reaction velocities for two enzymes: enzyme 1 with a small Michaelis constant (Km) and enzyme 2 with a large Km. Vmax = maximal velocity.
b. Large Km: A numerically large (high) Km reflects
a low affinity of enzyme for substrate because a high concentration of
substrate is needed to half-saturate the enzyme.
2. Relationship of velocity to enzyme
concentration: The
rate of the reaction is directly proportional to the enzyme concentration at
all substrate concentrations. For example, if the enzyme concentration is
halved, the initial rate of the reaction (vo), as well as that of
Vmax, are reduced to half that of the original.
3. Order of reaction: When [S] is much less than Km, the
velocity of the reaction is approximately proportional to the substrate
concentration (Figure 5.10). The rate of reaction is then said to be first
order with respect to substrate. When [S] is much greater than Km, the velocity
is constant and equal to Vmax. The rate of reaction is then independent of
substrate concentration (the enzyme is saturated with substrate) and is said to
be zero order with respect to substrate concentration (see Figure 5.10).
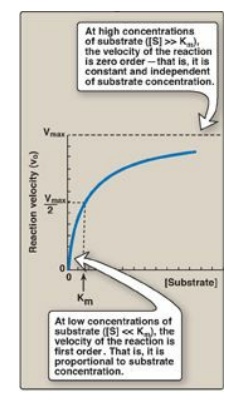
Figure 5.10 Effect of substrate concentration on reaction velocity for an enzymecatalyzed reaction. Vmax = maximal velocity; Km = Michaelis constant.
D. Lineweaver-Burk plot
When vo is
plotted against [S], it is not always possible to determine when Vmax
has been achieved because of the gradual upward slope of the hyperbolic curve
at high substrate concentrations. However, if 1/vo is plotted versus
1/[S], a straight line is obtained (Figure 5.11). This plot, the
Lineweaver-Burk reciprocal plot) can be used to calculate Km and Vmax
mechanism of action of enzyme inhibitors.
1. The equation
describing the Lineweaver-Burk plot is:
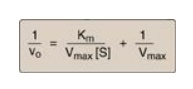
where the intercept on
the x axis is equal to −1/Km, and the intercept on the y axis is
equal to 1/Vmax. [Note: The slope = Km/Vmax.]
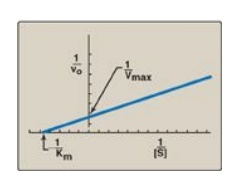
Figure 5.11 Lineweaver-Burk
plot. vo = reaction velocity; Vmax = maximal velocity; Km
= Michaelis constant; [S] = substrate concentration.
Related Topics
