Manufacture of Sterile Products
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Principles Of Good Manufacturing Practice
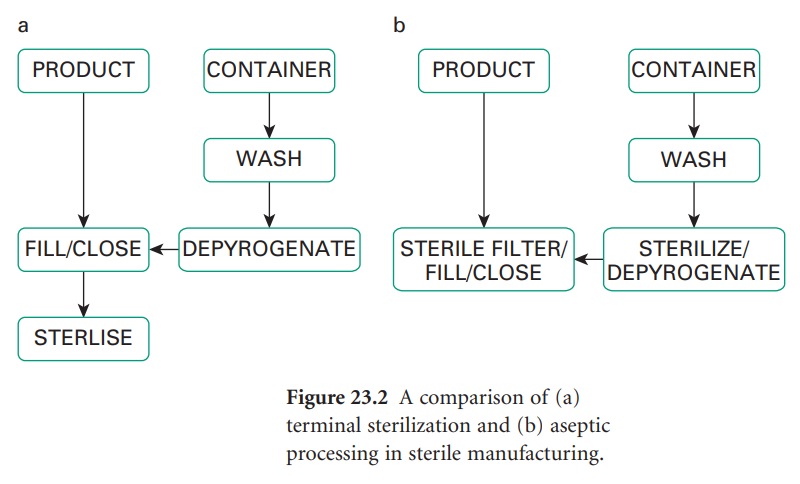
For production purposes an important distinction exists between sterile products which have been terminally sterilized and those which have not. Terminal sterilization involves the product being sealed in its container and then sterilized, usually by heat, but ionizing radiation or, less commonly, ethylene oxide may be employed.
MANUFACTURE OF
STERILE PRODUCTS
For production purposes an important distinction
exists between sterile products which have been
terminally sterilized (Figure
23.2a) and those which have not. Terminal
sterilization involves the
product being sealed in its container and then sterilized, usually by heat, but ionizing radiation or, less
commonly, ethylene
oxide may be employed. Such a product
must be manufactured in a clean
area. A product which cannot
be terminally sterilized is prepared aseptically
(Figure 23.2b) from
previously sterilized materials or by sterile filtration; in either case, aseptic filling
is a poststerilization step. Strict
aseptic conditions are
required throughout.
Vaccines, consisting of dead microorganisms, microbial extracts or inactivated viruses may be filled
in the same premises as other sterile
medicinal products, so the completeness of killing or removal of live organisms must be validated before
processing. Separate premises are needed for the filling
of live or attenuated vaccines and for the preparation of
other products derived from
live organisms. Non-sterile products and sterile
products must not be processed in the same area.
A) Clean And Aseptic Areas: General Requirements
i) Design of premises
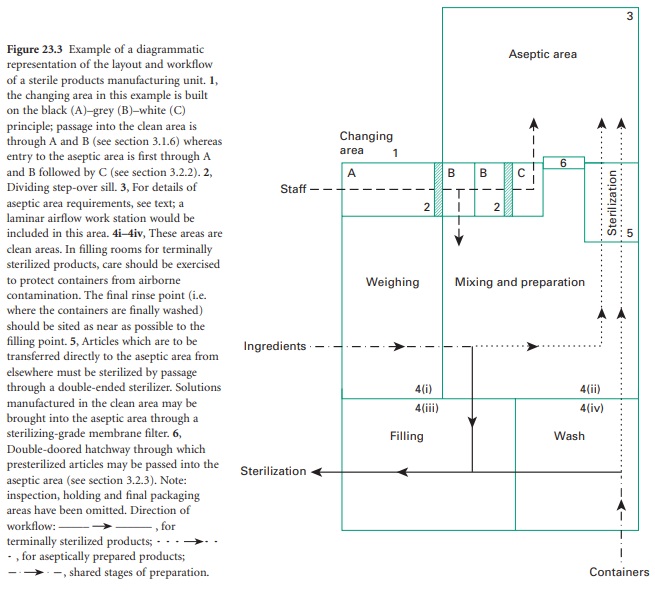
Sterile production should be carried
out in a purposebuilt unit separated
from other manufacturing areas and
thoroughfares. The unit should be designed to encourage
separation of each stage of production but should ensure a safe and organized workflow. A plan of such a facility
is shown in Figure
23.3. Sterilized products
held in quarantine pending sterility test results must be kept separate from those awaiting
sterilization.
ii) Internal Surfaces, fittings and floors
Particulate, as well as microbial,
contamination must be prevented.
To this end all surfaces must be smooth
and impervious in order
to: (1) prevent
accumulation of dust or other particulate matter; and (2) permit
easily repeated cleaning and disinfection. Smooth rounded coving should be used where the wall meets the floor and the ceiling.
Suitable flooring may be provided
by welded sheets
of PVC; cracks
and open joints which might harbour dirt and microorganisms must be avoided.
The preferred surfaces for walls
are plastic, epoxy-coated plaster, plastic
fibreglass or glass-reinforced polyester. Often the final finish for the floor, wall
and ceiling is achieved using continuous welded PVC sheeting. False ceilings should
be adequately sealed to prevent
contamination from the space above. Use should
be made of well-sealed glass panels, especially in dividing walls,
to ensure good visibility and allow satisfactory supervision. Doors and windows should be flush with the walls. Windows
should not be openable.
Internal fittings
such as cupboards, drawers and shelves should be kept
to a minimum. They must
be sited where they do not interfere with the laminar
flow of the
filtered air supply.
Stainless steel or laminated plastic
are the preferred materials for such fittings.
Stainless steel trolleys may be used to transport equipment
and materials within the clean and aseptic areas
but must remain confined to their respective units. Equipment must be
designed so that it may be easily
cleaned and sterilized or disinfected.
iii) Services
Clean and aseptic areas must be adequately illuminated; lights are best housed in translucent panels
set in a false ceiling. Electrical switches
and sockets must
be flush with the wall or fitted outside. When required, gases should be pumped in from
outside the unit.
Pipes and ducts,
if they must be brought
into the clean
area, must be sealed
through the walls. Additionally, in order to prevent dust accumulation,
pipes and ducts must be boxed in or
readily cleanable.
Alternatively, they may be sited above false ceilings.
Sinks should be of stainless steel with no overflow,
and water must
be of at least potable
quality. Wherever possible, drains should be avoided. If installed they must be fitted with effective, readily
cleanable traps and with air breaks to prevent
backflow. Any floor
channels should be open, shallow and cleanable and connected to drains outside the area; they should
be monitored microbiologically. Sinks and drains
should be excluded
from aseptic areas except where radiopharmaceuticals are being processed when sinks
are a requirement.
iv) Air Supply
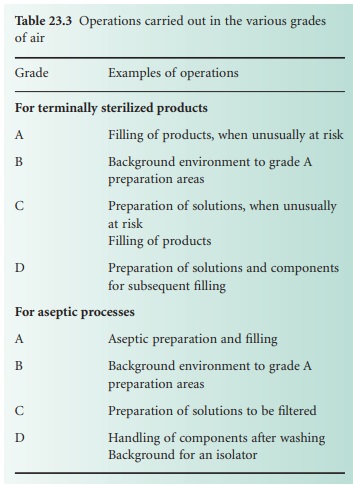
Areas for sterile
manufacture are classified according to the required characteristics of the environment. Each operation requires an appropriate level
of microbial and particulate cleanliness; four grades are specified in The
Rules and Guidance for Pharmaceutical Manufacturers and Distributors (2007). Environmental
quality is substantially influenced by the air supplied to the manufacturing environment. The grades of
air required for specific manufacturing activities are listed
in Table 23.3.
Filtered air is
used to achieve
the necessary standards; this should be maintained at positive pressure throughout a clean
or aseptic area, with the highest pressure in the most critical
rooms (aseptic or clean
filling rooms) and a progressive reduction through
the preparation and changing rooms (Figure 23.4); a minimum pressure
differential of 10 kPa is normally required between each class of room. A minimum of 20 changes of air per hour is usual in clean and aseptic
rooms. The air inlet points should
be situated in or
near the ceiling, with the final
filters placed as close as possible to the point
of input to the room.
Equipment or furnishings must be sited so as not to interfere with laminar flow.
The greatest risk of contamination of a product
comes from its immediate environment. Additional protection is needed both in the filling
area of the cleanroom and in
the aseptic suite. This can be provided by a workstation
supplied with a unidirectional flow
of filtered sterile
air. This is known as a laminar
flow cabinet. Displacement of air may be vertical or horizontal with
a typical homogeneous air flow of 0.45 m/s at the working
position. Consequently airborne
contamination is not added to the
work space, and any generated by manipulation is swept
away by the laminar
air currents. A fuller description of high efficiency particulate air (HEPA) filters in laminar
flow cabinets
is given by Gardner and
Peel (1998).
The efficacy
of the filters through which
the air is passed should be monitored at predetermined
intervals. Air quality
may be monitored for bacteria
and fungi by slit sampler or settle plate.
Particles are measured
using a discrete airborne particle
counter. The latest edition
of The Rules and Guidance for Pharmaceutical Manufacturers and Distributors (2007) states that particles must be monitored continuously in a grade A area and recommends it for grade
B areas. It should be noted that
grade A air is not the purest
that can be obtained; four
even cleaner grades are used in the electronics industry (ISO14644-1).
v)
Clothing
Clothing worn
in a clean area must
be of non-shedding fibres; polyester is a suitable
fabric. Airborne contamination, both microbial and particulate, is
reduced when trouser suits, close-fitting at the neck, wrists and ankles,
are worn.
Clean suits should
be provided once a day, but fresh headwear, overshoes and powder-free gloves are
necessary for each
working session. Special laundering facilities are desirable.
vi)
Changing facilities
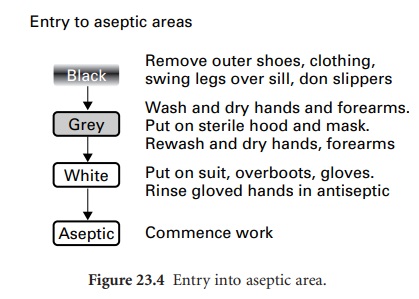
Entry to a clean or aseptic
area should be through a changing room fitted
with interlocking doors;
this design acts as an airlock to prevent influx
of air from
the outside. This route is for personnel only, not
for the transfer of materials and equipment. Staff entering the changing
room should already be clad in the standard
factory or hospital protective clothing. For entry
into a clean area, passage through
the changing room should be from
a ‘black’
to a ‘grey’ area, via a dividing
step-over sill (Figure 23.4). Movement
through these areas
and finally into the cleanroom
is permitted only when observing a strict protocol, whereby
outer garments are removed in the
‘black’ area and cleanroom trouser
suits donned in the
‘grey’ area. Only
after hand-washing in a sink fitted
with elbowor foot-operated taps may the operator
enter the cleanroom.
vii)
Cleaning
and disinfection
A strict,
validated disinfection policy
is necessary if microbial contamination is to be kept to a minimum.
Cleaning agents
used include alkaline
detergents and ionic and non-ionic surfactants. A wide range
of chemical disinfectants is available. Clear, soluble phenolics are commonly
used for interior
services and fittings. Disinfectants for working surfaces
are alcohols (70% ethanol or isopropanol) or, less commonly,
chlorine-based agents
such as hypochlorites. Skin may be disinfected with cationic detergents such as cetrimide
or chlorhexidine, usually
formulated with 70% alcohol to avoid
the need for rinsing. Gloved
hands may be disinfected with
these detergents or 70% alcohol. The former have the advantage of offering residual
activity. Rotation of different disinfectants reduces the risk of the emergence of resistant strains,
but such rotation
should be validated. In-use dilutions
must not be used unless
sterilized. Disinfectants and detergents for
use in grade A/B areas must be sterile
prior to use and formulated with water for injections. Modern
sprays are fitted
with devices to prevent air being sucked
back, extending the life of the
disinfectant. Smooth polished surfaces are more readily
cleaned. Floors and horizontal surfaces should
be cleaned and disinfected daily,
walls and ceilings
as often as required, but the interval should not exceed
1 month. Regular
microbiological monitoring should be carried out to determine
the efficacy of disinfection procedures. Records must be kept
and immediate remedial action taken should normal levels for that area be exceeded.
viii)
Operation
The number
of persons involved in sterile manufacture should be kept to a minimum to avoid the inevitable
turbulence and shedding of particles and organisms associated with the operatives. All operations should
be undertaken in a controlled and methodical manner as
excessive activity may increase turbulence and particle shedding.
Containers made
from fibrous materials such as paper,
cardboard and sacking are generally heavily
contaminated (especially with moulds and bacterial spores) and should not be taken into clean areas. Ingredients which must be brought
into clean areas must first be
transferred to suitable
metal or plastic containers. Containers and closures for terminally sterilized products must be thoroughly cleaned before
use and should undergo a final washing and rinsing process
in apyrogenic distilled
water (which has been passed
through a bacteria-proof membrane filter)
immediately prior to filling. Containers and closures for use in aseptic manufacture must, in addition,
be sterilized after washing and rinsing in preparation for aseptic filling.
Related Topics
