Hazard to Health
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Microbial Spoilage, Infection Risk And Contamination Control
Nowadays, it is well recognized that the inadvertent use of a contaminated pharmaceutical product may also present a potential health hazard to the patient. Although isolated outbreaks of medicament-related infections had been reported since the early part of the 20th century, it was only in the 1960s and 1970s that the significance of this contamination to the patient was more fully understood.
HAZARD TO HEALTH
Nowadays, it is well recognized that the inadvertent use of a
contaminated pharmaceutical product may also present a potential health hazard
to the patient. Although isolated outbreaks of medicament-related infections
had been reported since the early part of the 20th century, it was only in the
1960s and 1970s that the significance of this contamination to the patient was
more fully understood.
Inevitably, the infrequent isolation of true pathogens, such as Salmonella spp.
and the reporting of associated infections following the use of products
contaminated with these organisms (tablets with pancreatin and thyroid
extract), attracted considerable attention. More often, the isolation of common
saprophytic and non-fastidious opportunist contaminants with limited
pathogenicity to healthy individuals has presented a significant challenge to
compromised patients.
Gram-negative contaminants, particularly Pseudomonas spp.,
which have simple nutritional requirements and can multiply to significant
levels in aqueous products, have been held responsible for numerous outbreaks
of infection. For example, while the intact cornea is quite resistant to
infection, it offers little resistance to pseudomonads and related bacteria
when scratched, or damaged by irritant chemicals; loss of sight has frequently
occurred following the use of poorly designed ophthalmic solutions which had
become contaminated by Ps. aeruginosa and even supported its
active growth. Pseudomonads contaminating ‘antiseptic’ solutions have infected
the skin of badly burnt patients, resulting in the failure of skin grafts and
subsequent death from Gram-negative septicaemia. Infections of eczematous skin
and respiratory infections in neonates have been traced to ointments and creams
contaminated with Gram-negative bacteria. Oral mixtures and antacid suspensions
can support the growth of Gram-negative bacteria and serious consequences have
resulted following their inadvertent administration to patients who were
immuno-compromised as a result of antineoplastic chemotherapy. Growth of
Gram-negative bacteria in bladder washout solutions has been held responsible
for painful infections. In more recent times, Pseudomonas contamination
of TPN fluids during their aseptic compounding in the hospital pharmacy caused
the death of several children in the same hospital.
Fatal viral infections resulting from the use of contaminated human
tissue or fluids as components of medicines are well recorded. Examples of this
include HIV infection of haemophiliacs by contaminated and inadequately treated
factor VIII products made from pooled human blood, and Creutzfeldt–Jakob
disease (CJD) from injections of human growth hormone derived from human
pituitary glands, some of which were infected.
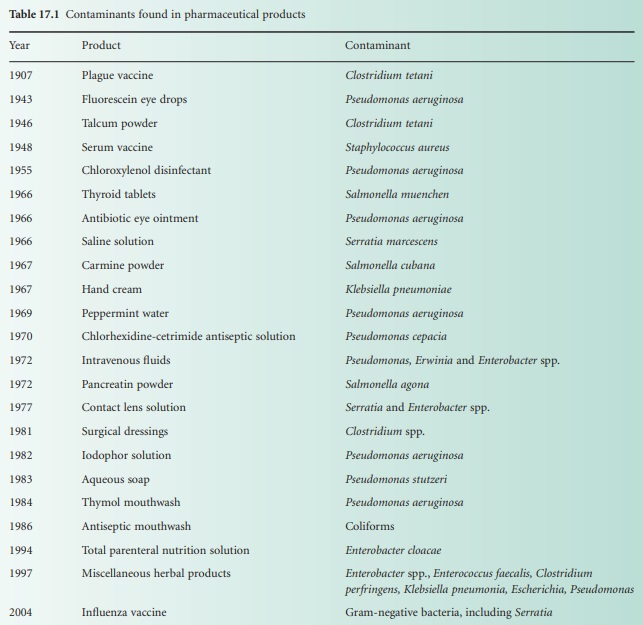
Pharmaceutical products of widely differing forms are known to be
susceptible to contamination with a variety of microorganisms, ranging from
true pathogens to a motley collection of opportunist pathogens (see Table 17.1). Disinfectants, antiseptics, powders, tablets and other products
providing an inhospitable environment to invading contaminants are known to be
at risk, as well as products with more nutritious components, such as creams
and lotions with carbohydrates, amino acids, vitamins and often appreciable
quantities of water.
The outcome of using a contaminated product may vary from patient to
patient, depending on the type and degree of contamination and how the product
is to be used. Undoubtedly, the most serious effects have been seen with
contaminated injected products where generalized bacteraemic shock and in some
cases death of patients have been reported. More likely, a wound or sore in
broken skin may become locally infected or colonized by the contaminant; this
may in turn result in extended hospital bed occupancy, with ensuing economic
consequences. It must be stressed, however, that the majority of cases of
medicament-related infections are probably not recognized or reported as such.
Recognition of these infections presents its own problems. It is a fortunate
hospital physician who can, at an early stage, recognize contamination shown as
a cluster of infections of rapid onset, such as that following the use of a
contaminated intravenous fluid in a hospital ward. The chances of a general
practitioner recognizing a medicament-related infection of insidious onset,
perhaps spread over several months, in a diverse group of patients in the
community, are much more remote. Once recognized, of course, there is a moral
obligation to withdraw the offending product; subsequent investigations of the
incident therefore become retrospective.
Microbial Toxins
Gram-negative bacteria contain lipo-polysaccharides (endotoxins) in their
outer cell membranes; these can remain in an active condition in products even
after cell death and some can survive moist heat sterilization. Although
inactive by the oral route, endotoxins can induce a number of physiological
effects if they enter the bloodstream via contaminated infusion fluids, even in
nanogram quantities, or via diffusion across membranes from contaminated
haemodialysis solutions. Such effects may include fever, activation of the
cytokine system, endothelial cell damage, all leading to septic and often fatal
febrile shock.
The acute bacterial toxins associated with food poisoning episodes are
not commonly reported in pharmaceutical products, although aflatoxin-producing
aspergilli have been detected in some vegetable and herbal ingredients.
However, many of the metabolites of microbial deterioration have quite
unpleasant tastes and smell even at low levels, and would deter most patients
from using such a medicine.
Related Topics
