Hammond Postulate
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Mechanisms of Organic Reactions
The relationship between energy of the transition state and the structure of the activated complex is summarized by the Hammond postulate, which states that the structure of the activated complex for any reaction step is most similar to the species (reactant or product) to which it is most similar in energy.
HAMMOND POSTULATE
The
relationship between energy of the transition state and the structure of the
activated complex is summarized by the Hammond postulate, which states that the
structure of the activated complex for any reaction step is most similar to the
species (reactant or product) to which it is most similar in energy. As seen
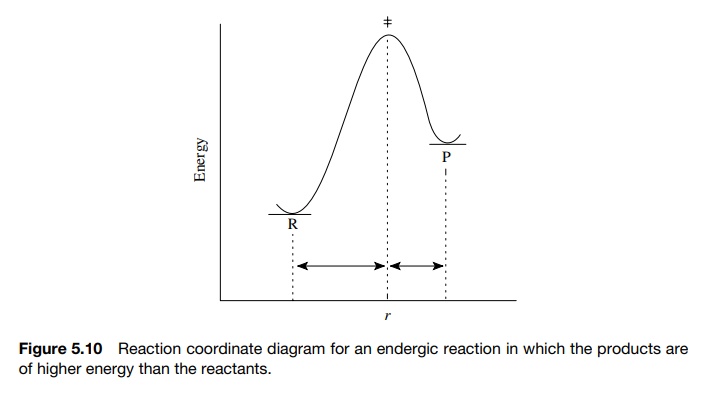
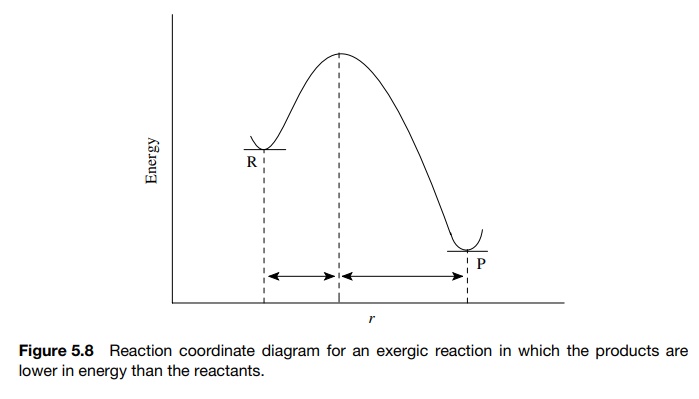
previously in Figure 5.8, exergic reactions (where the reactants are higher in
energy than the products) have early transition states and activated complexes
that resemble the reactants. Moreover, endergic reactions (in which the
products are higher in energy than the reactants) have late transition states
and activated complexes that resemble the products, as in Figure 5.10.
A
most useful application of the Hammond postulate involves reactions which
proceed by the formation of unstable intermediates, such as the carbocations,
The rate-determining step of such reactions
is necessarily endothermic, and the Hammond postulate serves as a use-ful tool
for identifying structural characteristics of the activated complex leading to
that intermediate. The logical next step is to ask how structural features in
reactants change the structure and thus energy of the activated complex.
The
Hammond postulate states that in endergic reactions, features which sta-bilize
and thus lower the energy of a product lower the energy of the transition state
leading to that product. This is shown in Figure 5.12. If product 2 (P2)
is lower in energy than product 1 (P1), then transition state 2 (±2) will be lower than
transition state 1 (±1). It will also be
earlier. As a consequence, P2 will have a lower activation barrier
and be formed faster than P1. A simplified restatement of the
Hammond postulate is that more stable products are formed faster. It must be
remembered that this analysis is for endothermic reactions and assumes that the
reactants have the same or similar energies.

The
ionization of alkyl tosylates to give carbocations is an endothermic reac-tion.
Knowing that 3◦
carbocations are more stable than 2◦ carbocations, we would conclude
that the activation barrier for ionization of the 3◦ tosylate is lower
than that of the 2◦
tosylate, and thus the 3◦
tosylate should ionize faster (Figure 5.13).

We
would also predict that the transition state for ionization of the 3◦ tosylate would be
earlier, so there should be less C–O bond breaking and less charge development
than in the activated complex for ionization of the 2◦ tosylate.
The
Hammond postulate provides a key relationship between the rate of reaction and
the activated complex of that reaction (Figure 5.14). In practice, structural
changes are made in the reactant(s) and the influence of those changes on the
rate of reaction is measured. If the reaction is faster, then the change in the

If the
reac-tion is slower, then the change in the reactant has led to a higher
product energy and hence a higher activation energy and a later transition
state (one with more product character). The results of rate studies can thus
be translated into structural changes (bonding, charge distribution, geometry)
in the activated complex, which further translates into the mechanistic
information about the reaction.
The
Hammond postulate is best applied to reactions with unstable intermedi-ates,
such as the carbocations in the above example. In such cases the transition
state is late and the activated complex more resembles the intermediate. Thus
changes in the energy of the intermediate have the greatest effect on the
energy of the transition state and thus the rate of the reaction.
However,
the Hammond postulate also holds for exergic reactions where the transition
state is early and the activated complex more resembles the reactant. For such
reactions changes in the energy of the reactants have the greatest effect on
the energy of the transition state and thus the rate of the reaction.
Related Topics
