Electronic structures of atoms
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Introduction
It is important to understand the basic inorganic principles in order to evaluate the full potential of inorganic compounds in clinical applications.
Basic inorganic principles
It is important to understand the basic inorganic principles in
order to evaluate the full potential of inorganic compounds in clinical
applications. In the following sections, aspects such as atomic structures,
chemical bonds and the set-up of the periodic table will be discussed.
Electronic
structures of atoms
1. What is an atom
An atom is defined as the smallest unit that retains the
properties of an element. The most famous definition has been published by
Dalton in his Atomic Theory :
All
matter is composed of atoms and these cannot be made or destroyed. All atoms of the same element are identical
and different elements have different types of atoms. Chemical reactions occur when atoms are rearranged .
After Dalton’s time, research showed that atoms actually can be
broken into smaller particles, and with the help of nuclear processes it is
even possible to transform atoms. Nevertheless, these processes are not
necessarily considered as chemical processes. Probably, a better definition is
that atoms are units that cannot be created, destroyed or transformed into other
atoms in a chemical reaction.
Atoms consist of three fundamental types of particles: protons,
electrons and neutrons. Neutrons and pro-tons have approximately the same mass
and, in contrast to this, the mass of an electron is negligible. A proton
carries a positive charge, a neutron has no charge and an electron is
negatively charged. An atom contains equal numbers of protons and electrons and
therefore, overall, an atom has no charge. The nucleus of an atom contains
protons and neutrons only, and therefore is positively charged. The electrons
occupy the region of space around the nucleus. Therefore, most of the mass is
concentrated within the nucleus.
Figure 1.3 shows the typical shorthand writing method for elements,
which can also be found in most periodic tables of elements. Z (atomic number) represents the number
of protons and also electrons, as an element has no charge. The letter A stands for the mass number, which
represents the number of protons and neutrons in the nucleus. The number of
neutrons can be determined by calculating the difference between the mass
number (A) and the atomic number (Z).
Within an element, the atomic number (Z), that is, the number of protons and electrons, is always the
same, but the number of neutrons and therefore the mass number (A) can vary. These possible versions of
an element are called isotopes.
Further discussion on radioisotopes and radioactivity can be found in Chapter
10.
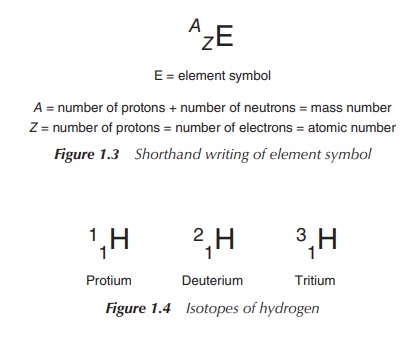
Atoms
of the same element can have different numbers of neutrons; the different
possible versions of each element are called isotopes. The numbers of protons and electrons are the same for
each isotope, as they define the element and its chemical behaviour.
For example, the most common isotope of hydrogen called protium has no neutrons at all. There
are two other hydrogen isotopes: deuterium, with one neutron, and tritium, with
two neutrons (Figure 1.4).
2. Bohr model of atoms
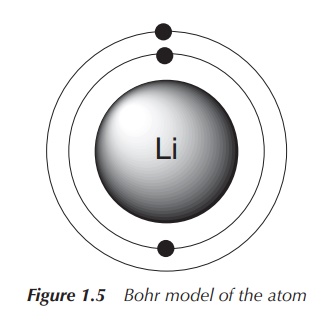
In 1913, Niels Bohr published his atomic model stating that
electrons can only circle the nucleus on fixed orbits in which the electron has
a fixed angular momentum. Each of these orbits has a certain radius (i.e.
distance from the nucleus), which is proportional to its energy. Electrons
therefore can only change between the fixed energy levels (quantisation of
energy), which can be seen as light emission. These fixed energy levels are
defined as the principal quantum number n,
which is the only quantum number introduced by the Bohr model of the atom. Note
that, as the value of n increases,
the electron is further away from the nucleus. The further away the electron is
from the nucleus, the less tightly bound the electron is to the nucleus (Figure
1.5).
3. Wave mechanics
In 1924, Louis de Brogli argued that all moving particles,
especially electrons, show a certain degree of wave-like behaviour. Therefore,
he proposed the idea of wave-like nature of electrons, which became known as
the phenomenon of the wave–particle
duality .
Schrödinger published in 1926 the famous wave equation named after him. Electrons are described as wave functions rather than defined particles. Using this approach, it was possible to explain the unanswered questions from Bohr’s model of the atom. Nevertheless, if an electron has a wave-like consistency, there are important and possibly difficult-to-understand consequences; it is not possible to determine the exact momen-tum and the exact position at the same moment in time. This is known as Heisenberg’s Uncertainty Principle. In order to circumvent this problem, the probability of finding the electron in a given volume of space is used.
The Schrödinger wave equation delivers information about the
wave function, and it can be solved either exactly or approximately. Only
hydrogen-like atoms or ions, that is, the ones containing a nucleus and only
one electron, can be exactly solved with the Schrödinger wave equation. For all
other atoms or ions, the equation can be solved only approximately.
Solving the Schrödinger wave function gives us information about
(i) the region or volume of space where the electron is most likely to be
found, that is, where the probability of finding the electron is highest. This
volume of space is called an atomic
orbital (AO), which is defined by a wave function. (ii) Energy values associated with particular
wave functions can be obtained by solving the Schrödinger wave equation. (iii)
It can be shown that there is a quantisation
of energy levels, similar to the observation described by Bohr.
4. Atomic orbitals
Each AO is defined by three so-called quantum numbers (n, l,
ml):
The principal quantum number n
has already been introduced with the Bohr model of atoms. It can take values of
1 ≤ n ≤ ∞, and is the result
of the radial part of the wave function being solved.
Each
atomic orbital is defined by a set of three
quantum numbers: the principal quantum number (n), the orbital quantum
number (l) and the magnetic quantum number (ml).
The quantum numbers l
and ml are obtained when
the angular part of the wave function is solved. The quantum number l represents the shape of the AO. It is
called the orbital quantum number as
it represents the orbital angular momentum of the electron. It can have values
of l = 0, 1, 2, … , (n − 1), which correspond to the orbital
labels s, p, d and f (see Figure 1.6).
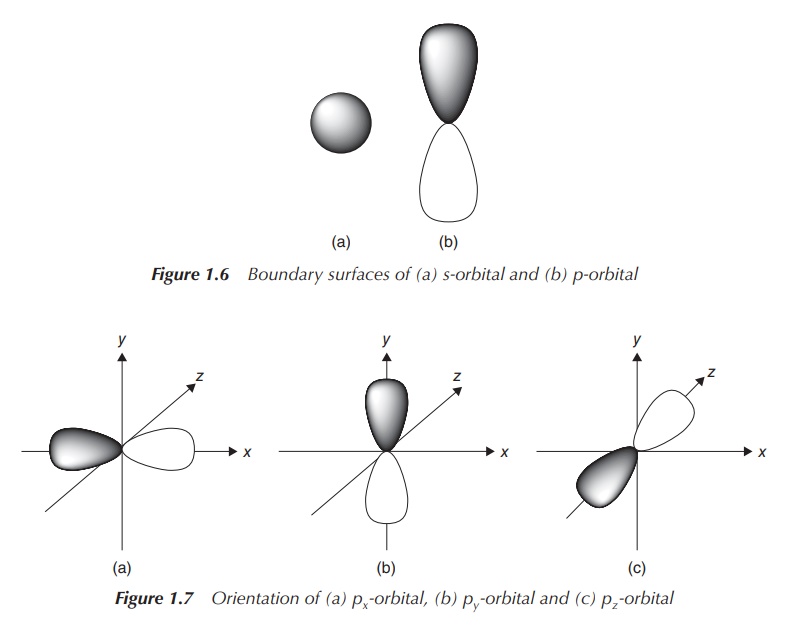
The magnetic quantum number ml
provides information about the orientation (directionality) of the AO and can
take values between +l and −l. This means that there is only one
direction for an s-orbital, as l = 0,
and therefore ml also is
equal to 0. For a p-orbital, l = 1
and therefore ml is −1, 0
or 1, which means it can occupy three orientations. In this case, they are
classified as the px, py and pz orbitals (see Figure 1.7). In the case of a d orbital
(l = 2), the quantum number ml = −2, −1, 0, 1 or 2.
Therefore, there are five d orbitals with different orientations (see Figure
1.8).
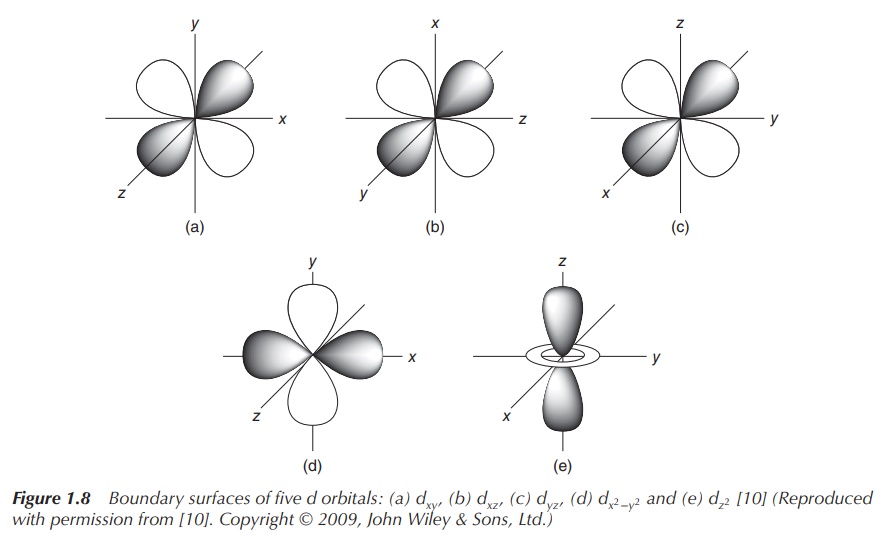
The state of each individual electron can be described by an additional fourth quantum number, the so-called spin quantum number s (value of either +1/2 or −1/2). Each orbital can be filled with one or two electrons. Once an orbital is filled with two electrons, they will occupy opposite spin directions in order to fulfil thePauli Exclusion Principle .
The Pauli Exclusion Principle states that
no two electrons in the same atom can have the same values for their four
quantum numbers.
5. Electron configuration and Aufbau principle
Each element in the periodic table is characterised by a set of
electrons, and their configuration can be described with the help of the
quantum numbers that have been introduced in Section 1.2.1.4.
The electronic configuration is mostly used to describe the
orbitals of an atom in its ground state and shows how these are distributed
between the different orbitals. Nevertheless, this model can also be used to
show valence electrons or ions.
A valence electron is defined as an
electron that is part of an atom and can participate in the formation of a chemical
bond. In main group elements, the valence electron is positioned in the
outermost shell.
The principle of electron configuration of an atom was already
established with the Bohr model of atoms, and therefore very often the terms
‘shell’ and ‘subshell’ are used. In this nomenclature, the electron shell
describes a set of electrons that occupy the same principal quantum number n. The respective electron shell n can be filled with 2n2 electrons. This means the first electron shell can be filled with
a maximum of two electrons, as n = 1. Note that, according to the Pauli Exclusion Principle, these two
electrons have an opposite spin direction. The second electron shell can
accommodate up to eight electrons with n = 2, and so on. The subshells are defined
by the quantum number l (l =
n − 1), which as previously
described correspond to the orbital
labels s, p, d and f. The number of electrons that can be placed in each
subshell can be determined by the following equation: 2(2l + 1). This allows two electrons to be placed in the s subshell (l = 0), six electrons in the p subshell
(l = 1) and 10 electrons in the d
subshell (l = 2). This information
can be translated into the occupation of the corresponding orbitals. There are
three p orbitals with differing ori-entations as defined by the quantum number ml. Each of those can
accommodate two electrons (which will occupy opposite spin directions as
explained above). This means the three p orbitals can be filled with six
electrons in total (Figure 1.9).
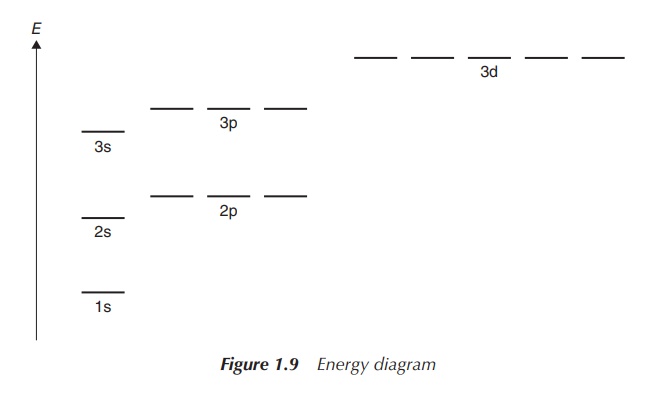
The so-called Aufbau
principle (German for ‘building-up principle’) helps us to determine the
electron configuration of an atom. It describes the hypothetical process of
filling the orbitals of an atom with the given number of electrons. The first
orbitals filled are the ones with the lowest energy levels before going onto
the next higher energy level. This means the 1s orbital is filled before the 2s
orbital. According to the so-called Hund’s
Rule, orbitals of the same energy level (such as p or d orbitals) are
filled with one electron first before the
electrons are paired within the same orbital. Pairing electrons requires
additional energy, as the spin of the second electron has to be reversed in
order obey the Pauli Exclusion Principle.
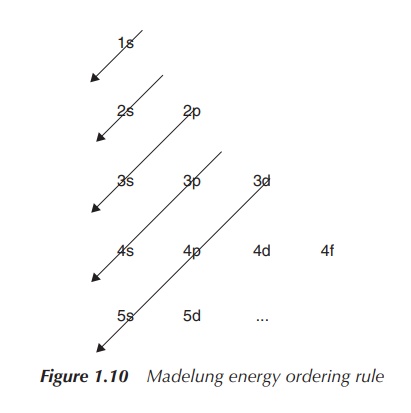
The Madelung Energy Ordering Rule helps us to determine
which orbitals are filled first. Orbitals are arranged by increasing energy, which means that order of occupation of
the relevant orbitals is visualised by the arrow. Note that there are
exceptions to this rule, which can be seen especially for electron
configurations where d and/or f orbitals are occupied (Figure 1.10).
Scientists use the following standard notation to indicate the electronic configuration of an atom: Basically, the sequence of the occupied orbitals is written in order, with the number of electrons occupying each orbital as superscript number. In the simplest case hydrogen (H), which has only one electron occupying the 1s orbital, the electronic configuration is written as 1s1.
Helium (He) has two electrons in the 1s orbital and therefore its electronic
configuration can be denoted as 1s2. Lithium (Li) has three
electrons in total, and its electron con-figuration is 1s22s 1.
A more complicated example is nitrogen (N), which has seven electrons, and its
electronic configuration can be written as 1s12s22p3.
When more electrons are involved, the electronic configurations can get
increasingly complicated. Therefore, lengthy notations can be shortened if some
of the subshells are identical to those of noble gases. Sulfur (S) has the
electronic configuration 1s22s22p63s23p4.
The first three subshells (underlined) are identical to the electronic
configuration of the noble gas neon (1s22s22p6),
which has all subshells completely filled, as this is characteristic for noble
gases. The shortened notation for the electronic configuration of sulfur can
therefore be written as [Ne]3s23p4, where [Ne] is the
short form for the electron configuration of neon.
Noble gas configuration is the term used for the description of the
electronic configuration of noble gases.
This notation is also useful to identify the valence electrons of an atom, which are located in the outer shells. These electrons typically determine the chemical behaviour, as elements strive to achieve the noble gas configuration by gaining or removing electrons from these shells.
Related Topics
