Electron Transport Chain
| Home | | Biochemistry |Chapter: Biochemistry : Bioenergetics and Oxidative Phosphorylation
Energy-rich molecules, such as glucose, are metabolized by a series of oxidation reactions ultimately yielding CO2 and water.
ELECTRON TRANSPORT CHAIN
Energy-rich molecules,
such as glucose, are metabolized by a series of oxidation reactions ultimately
yielding CO2 and water (Figure 6.6). The metabolic intermediates of
these reactions donate electrons to specific coenzymes, nicotinamide adenine
dinucleotide (NAD+) and flavin adenine dinucleotide (FAD), to form
the energy-rich reduced forms, NADH and FADH2. These reduced
coenzymes can, in turn, each donate a pair of electrons to a specialized set of
electron carriers, collectively called the electron transport chain (ETC),
described in this section. As electrons are passed down the ETC, they lose much
of their free energy. This energy is used to move protons across the inner
mitochondrial membrane, creating a proton gradient that drives the production
of ATP from ADP and inorganic phosphate (Pi). The coupling of electron
transport with ATP synthesis is called oxidative phosphorylation, often denoted
as OXPHOS. It proceeds continuously in all tissues that contain mitochondria.
[Note: The remainder of the free energy not trapped as ATP is used to drive
ancillary reactions such as calcium transport into mitochondria and to generate
heat.]
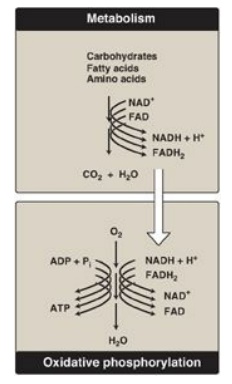
Figure 6.6 The metabolic breakdown of energyyielding molecules. NAD(H) = nicotinamide adenine dinucleotide; FAD(H2)= flavin adenine dinucleotide; ADP = adenosine diphosphate; ATP = adenosine triphosphate; Pi = inorganic phosphate.
A. The electron transport chain of the mitochondrion
The ETC (except for
cytochrome c;) is located in the inner mitochondrial membrane and is the final
common pathway by which electrons derived from different fuels of the body flow
to oxygen (O2).
1. Membranes of the mitochondrion: The mitochondrion contains an outer and an inner membrane separated by the intermembrane space. Although the outer membrane contains special channels (formed by the protein porin), making it freely permeable to most ions and small molecules, the inner membrane is a specialized structure that is impermeable to most small ions, including protons and small molecules such as ATP, ADP, pyruvate, and other metabolites important to mitochondrial function (Figure 6.7). Specialized carriers or transport systems are required to move ions or molecules across this membrane. The inner mitochondrial membrane is unusually rich in protein, over half of which is directly involved in oxidative phosphorylation. It also is highly convoluted. The convolutions, called cristae, serve to greatly increase the surface area of the inner membrane.
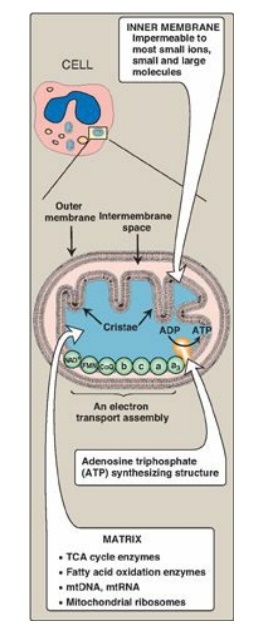
Figure 6.7 Structure of a mitochondrion showing schematic representation of the electron transport chain and the ATP synthesizing structure on the inner membrane. [Note: In contrast to the inner membrane, the outer membrane is highly permeable, and the milieu of the intermembrane space is like that of the cytosol.] mtDNA = mitochondrial DNA; mtRNA = mitochondrial RNA; TCA = tricarboxylic acid.
2. Matrix of the mitochondrion: This gel-like solution in the interior of mitochondria is also rich in protein. These molecules include the enzymes responsible for the oxidation of pyruvate, amino acids, and fatty acids (by β-oxidation) as well as those of the tricarboxylic acid (TCA) cycle. The synthesis of glucose, urea, and heme occurs partially in the matrix of mitochondria. In addition, the matrix contains NAD+ and FAD (the oxidized forms of the two coenzymes that are required as hydrogen acceptors), and ADP and Pi, which are used to produce ATP. [Note: The matrix also contains mitochondrial DNA (mtDNA) and RNA (mtRNA) and ribosomes.]
B. Organization of the electron transport chain
The inner mitochondrial
membrane contains five separate protein complexes, called Complexes I, II, III,
IV, and V. Complexes I–IV each contain part of the ETC ( Figure 6.8). These
complexes accept or donate electrons to the relatively mobile electron
carriers, coenzyme Q and cytochrome c. Each carrier in the ETC can receive
electrons from an electron donor and can subsequently donate electrons to the
next acceptor in the chain. The electrons ultimately combine with O2
and protons to form water. This requirement for O2 makes the
electron transport process the respiratory chain, which accounts for the
greatest portion of the body’s use of O2.
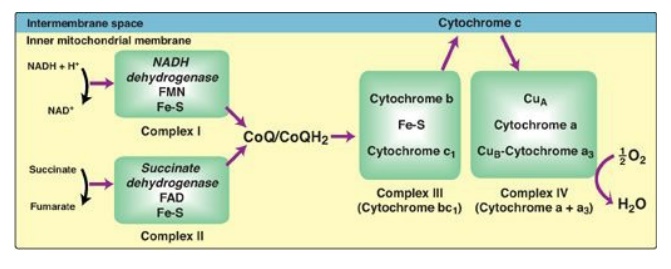
Figure 6.8 Electron transport chain. The flow of electrons is shown by the magenta arrows. NAD(H) = nicotinamide adenine dinucleotide; FMN = flavin mononucleotide; FAD = flavin adenine dinucleotide; Fe-S = iron-sulfur center; CoQ = coenzyme Q.
C. Reactions of the electron transport chain
With the exception of
coenzyme Q, which is a lipid-soluble quinone, all members of this chain are
proteins. These may function as enzymes as is the case with the
flavin-containing dehydrogenases, may contain iron as part of an iron-sulfur
center, may contain iron as part of the porphyrin prosthetic group of heme as
in the cytochromes, or may contain copper as does the cytochrome a + a3
complex.
1. Formation of NADH: NAD+ is reduced to NADH
by dehydrogenases that remove two hydrogen atoms from their substrate. (For
examples of these reactions, see the discussion of the dehydrogenases of the
TCA cycle) Both electrons but only one proton (that is, a hydride ion [:H–])
are transferred to the NAD+, forming NADH plus a free proton.
2. NADH dehydrogenase: The free proton plus the hydride ion carried by NADH are transferred to NADH dehydrogenase, a protein complex (Complex I) embedded in the inner mitochondrial membrane. Complex I has a tightly bound molecule of flavin mononucleotide (FMN), a coenzyme structurally related to FAD (see Figure 28.15. that accepts the two hydrogen atoms (2e–I + I 2H+), becoming FMNH2. NADH dehydrogenase also contains peptide subunits with iron-sulfur centers (Figure 6.9). At Complex I, electrons move from NADH to FMN to the iron of the iron-sulfur centers and then to coenzyme Q. As electrons flow, they lose energy. This energy is used to pump protons across the inner mitochondrial membrane, from the matrix to the intermembrane space.
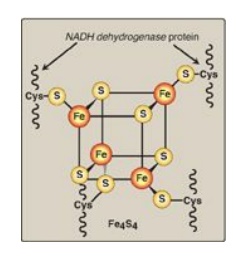
Figure 6.9 Iron-sulfur (Fe-S) center of Complex I. [Note: Complexes II and III also contain iron-sulfur centers.] NADH = nicotinamide adenine dinucleotide;Cys = cysteine.
3. Succinate dehydrogenase: At Complex II, electrons from the succinate dehydrogenase–catalyzed oxidation of succinate to fumarate move from the coenzyme, FADH2, to an iron-sulfur protein, and then to coenzyme Q. [Note: No energy is lost in this process, and, therefore, no protons are pumped at Complex II.]
4. Coenzyme Q: Coenzyme Q (CoQ) is a quinone derivative with a
long, hydrophobic isoprenoid tail. It is also called ubiquinone because it is
ubiquitous in biologic systems. CoQ is a mobile electron carrier and can accept
hydrogen atoms from NADH dehydrogenase (Complex I), from succinate
dehydrogenase (Complex II), and from other mitochondrial dehydrogenases: glycerophosphate
dehydrogenase and acyl CoA dehydrogenase. CoQ transfers electrons to Complex
III (cytochrome bc1). CoQ, then, links the flavoprotein dehydrogenases to the
cytochromes.
5. Cytochromes: The remaining members of the ETC are cytochrome
proteins. Each contains a heme group (a porphyrin ring plus iron). Unlike the
heme groups of hemoglobin, the cytochrome iron is reversibly converted from its
ferric (Fe3+) to its ferrous (Fe2+) form as a normal part
of its function as an acceptor and donor of electrons. Electrons are passed
along the chain from cytochrome bc1 (Complex III), to cytochrome c,
and then to cytochromes a + a3 (Complex IV; see Figure 6.8). As
electrons flow, protons are pumped across the inner mitochondrial membrane at
Complexes III and IV. [Note: Cytochrome c is located in the intermembrane
space, loosely associated with the outer face of the inner membrane. As seen
with CoQ, cytochrome c is a mobile carrier of electrons.]
6. Cytochrome a + a3: This cytochrome complex (Complex IV) is the only electron carrier in which the heme iron has an available coordination site that can react directly with O2 and so also is called cytochrome oxidase. At Complex IV, the transported electrons, O2, and free protons are brought together, and O2 is reduced to water (see Figure 6.8). [Note: Four electrons are required to reduce one molecule of O2 to two molecules of water.] Cytochrome oxidase contains copper (Cu) atoms that are required for this complicated reaction to occur. Electrons move from CuA to cytochrome a to cytochrome a3 (in association with CuB) to O2.
7. Site-specific inhibitors: Site-specific inhibitors of
electron transport have been identified and are illustrated in Figure 6.10.
These compounds prevent the passage of electrons by binding to a component of
the chain, blocking the oxidation-reduction reaction. Therefore, all electron
carriers before the block are fully reduced, whereas those located after the
block are oxidized. [Note: Inhibition of electron transport inhibits ATP
synthesis because these processes are tightly coupled.]
Incomplete reduction of oxygen to water produces
reactive oxygen species (ROS), such as superoxide (O2–•),
hydrogen peroxide (H2O2), and hydroxyl radicals (OH•).
ROS damage DNA and proteins and cause lipid peroxidation. Enzymes such as
superoxide dismutase (SOD) , catalase, and glutathione peroxidase are cellular
defenses against ROS.
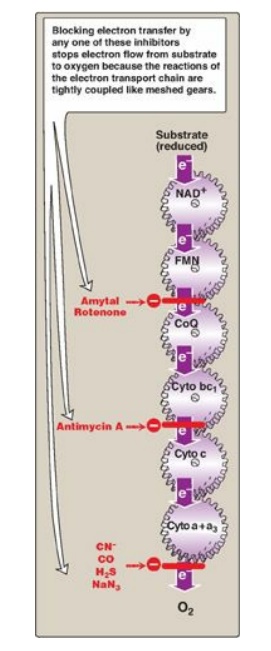
Figure 6.10 Site-specific
inhibitors of electron transport shown using a mechanical model for the
coupling of oxidationreduction reactions. [Note: Figure illustrates normal
direction of electron flow.] CN- = cyanide; CO = carbon monoxide; H2S
= hydrogen sulfide; NaN3 = sodium azide; FMN = flavin
mononucleotide; FAD = flavin adenine dinucleotide; CoQ = coenzyme Q; Cyto =
cytochrome.
D. Release of free energy during electron transport
The free energy
released as electrons are transferred along the ETC from an electron donor
(reducing agent or reductant) to an electron acceptor (oxidizing agent or
oxidant) is used to pump protons at Complexes I, III, and IV. [Note: The
electrons can be transferred as hydride ions (:H–) to NAD+;
as hydrogen atoms (•H) to FMN, CoQ, and FAD; or as electrons (e–) to
cytochromes.]
1. Redox pairs: Oxidation (loss of electrons) of one substance is
always accompanied by reduction (gain of electrons) of a second. For example,
Figure 6.11 shows the oxidation of NADH to NAD+ by NADH dehydrogenase
at Complex I, accompanied by the reduction of FMN, the prosthetic group, to FMNH2.
Such oxidation-reduction reactions can be written as the sum of two separate
half-reactions, one an oxidation and the other a reduction (see Figure 6.11). NAD+
and NADH form a redox pair, as do FMN and FMNH2. Redox pairs differ
in their tendency to lose electrons. This tendency is a characteristic of a
particular redox pair and can be quantitatively specified by a constant, Eo
(the standard reduction potential), with units in volts.
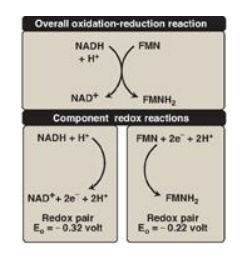
Figure 6.11 Oxidation of NADH by FMN, separated into two component half-reactions. NAD(H) = nicotinamide adenine dinucleotide; FMN(H2) = flavin mononucleotide.
2. Standard reduction potential: The Eo of various redox
pairs can be ordered from the most negative Eo to the most positive.
The more negative the Eo of a redox pair, the greater the tendency
of the reductant member of that pair to lose electrons. The more positive the Eo,
the greater the tendency of the oxidant member of that pair to accept
electrons. Therefore, electrons flow from the pair with the more negative Eo
to that with the more positive Eo. The Eo values for some
members of the ETC are shown in Figure 6.12. [Note: The components of the chain
are arranged in order of increasingly positive Eo values.]
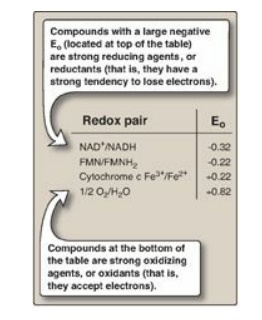
Figure 6.12 Standard
reduction potentials (Eo) of some reactions. NAD(H) = nicotinamide adenine
dinucleotide; FMN(H2) = flavin mononucleotide.
3. Relationship of ∆Go to ∆EοI
: The ∆Go
is related directly to the magnitude of the change in Eo:
∆Go = – n F ∆Eo
where
n = number of electrons
transferred (1 for a cytochrome, 2 for NADH, FADH2, and coenzyme Q)
F = Faraday constant (23.1 kcal/volt mol)
∆Eo = Eo of the electron-accepting pair minus
the Eo of the electron-donating pair
∆Go = change in the standard free energy
4. ∆Go of ATP: The ∆Go for the phosphorylation of ADP to ATP is +7.3 kcal/mol. The transport of a pair of electrons from NADH to O2 through the ETC releases 52.58 kcal. Therefore, more than sufficient energy is available to produce 3 ATP from 3 ADP and 3 Pi (3 × 7.3 = 21.9 kcal/mol), sometimes expressed as a P:O ratio (ATP made per O atom reduced) of 3:1. The remaining calories are used for ancillary reactions or released as heat. [Note: The P:O for FADH2 is 2:1 because Complex I is bypassed.]
Related Topics
