Disinfection Policies
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Chemical Disinfectants, Antiseptics And Preservatives
The aim of a disinfection policy is to control the use of chemicals for disinfection and antisepsis and give guidelines on their use.
DISINFECTION POLICIES
The aim of a disinfection policy
is to control the use of
chemicals for disinfection and antisepsis and give guidelines on their use. The preceding descriptions within this article of the activities, advantages and disadvantages of the many disinfectants available allow considerable scope for choice and
inclusion of agents
in a policy to be applied
to such
areas as industrial plant, walls, ceilings, floors, air, cleaning equipment and laundries and
to the extensive range of equipment in contact with hospital patients.
The control of microorganisms is of prime importance in hospital and industrial environments. Where pharmaceutical products (either sterile or non-sterile) are manufactured, contamination of the product may lead to its deterioration and to infection in the user. In hospital there is the additional consideration of patient care, therefore protection from nosocomial (hospital-acquired) infection and prevention of cross-infection must also be covered. Hospitals will have a disinfection policy and the degree of adherence to, and implementation of, the policy content will require stringent monitoring. A specialized infection control committee or similar, comprising a number of specialized personnel such as the pharmacist, the consultant medical microbiologist and senior nurse responsible for infection control, should formulate a suitable policy. This core team may usefully be expanded to include, for example, a physician, a surgeon, nurse teachers and nurses from several clinical areas, the sterile services manager and the domestic superintendent; purchasing. This expanded committee will meet regularly to help with the implementation of the policy and reassess its efficiency. Tables 19.2–19.4 indicate the susceptibility of various microorganisms to the range of agents available and Table 19.6 presents examples of the range of formulations available. Although scope exists for choice of disinfectant in many of the areas covered by a policy, in certain instances specific recommendations are made as to the type, concentration and usage of disinfectant.
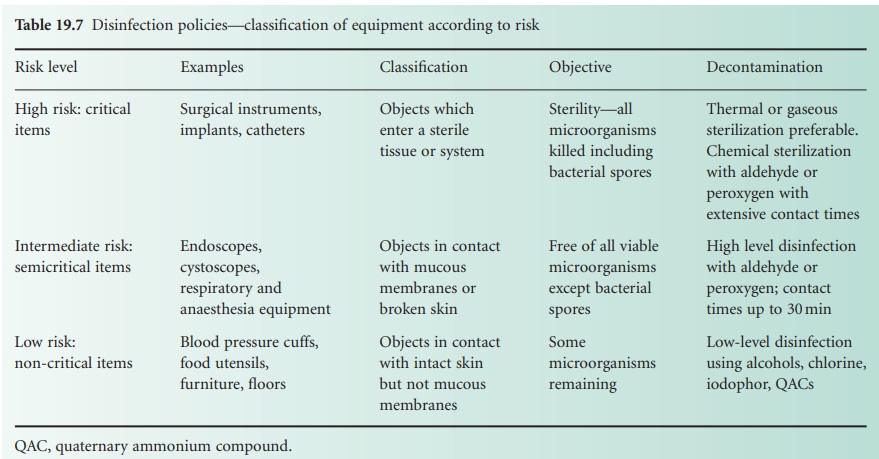
Categories of risk (to patients) may
be assigned to equipment coming into
contact with a patient, dictating the level of decontamination required
and degree of concern (Table 19.7). High-risk (critical) items have close
contact with broken skin or mucous membranes or are those introduced into a sterile
area of the body and should, themselves, be sterile; they include instruments, gloves, catheters, syringes
and needles. Liquid chemical disinfectants
should only be used if heat or other methods of sterilization are unsuitable. Intermediate-risk (semicritical) items are in close
contact with skin or mucous membranes and disinfection will normally be applied.
Endoscopes, respiratory and anaesthetic equipment, wash bowls, bed-pans and similar items
are included in this category. Low-risk (non-critical) items or areas include those detailed
earlier such as walls and floors,
which are not in close
contact with the patient. Cleaning is obviously important with
disinfection being required, for example, in the event
of contaminated spillage.
Related Topics
