Degradation of Glycogen (Glycogenolysis)
| Home | | Biochemistry |Chapter: Biochemistry : Glycogen Metabolism
The degradative pathway that mobilizes stored glycogen in liver and skeletal muscle is not a reversal of the synthetic reactions.
DEGRADATION OF GLYCOGEN (GLYCOGENOLYSIS)
The degradative pathway
that mobilizes stored glycogen in liver and skeletal muscle is not a reversal
of the synthetic reactions. Instead, a separate set of cytosolic enzymes is
required. When glycogen is degraded, the primary product is glucose
1-phosphate, obtained by breaking α(1→4) glycosidic bonds. In addition, free
glucose is released from each α(1→6)–linked glucosyl residue (branch point).
A. Shortening of chains
Glycogen phosphorylase
sequentially cleaves the α(1→4) glycosidic bonds between the glucosyl residues
at the nonreducing ends of the glycogen chains by simple phosphorolysis
(producing glucose 1-phosphate) until four glucosyl units remain on each chain
before a branch point (Figure 11.7). [Note: Phosphorylase contains a molecule
of covalently bound pyridoxal phosphate that is required as a coenzyme.] The
resulting structure is called a limit dextrin, and phosphorylase cannot degrade
it any further (Figure 11.8).
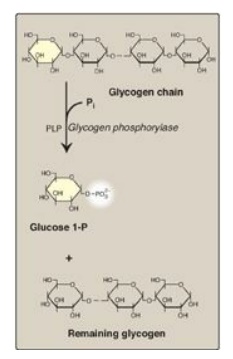
Figure 11.7 Cleavage of an α(1→4)-glycosidic bond. PLP= pyridoxal phosphate; Pi = inorganic phosphate; P = phosphate.
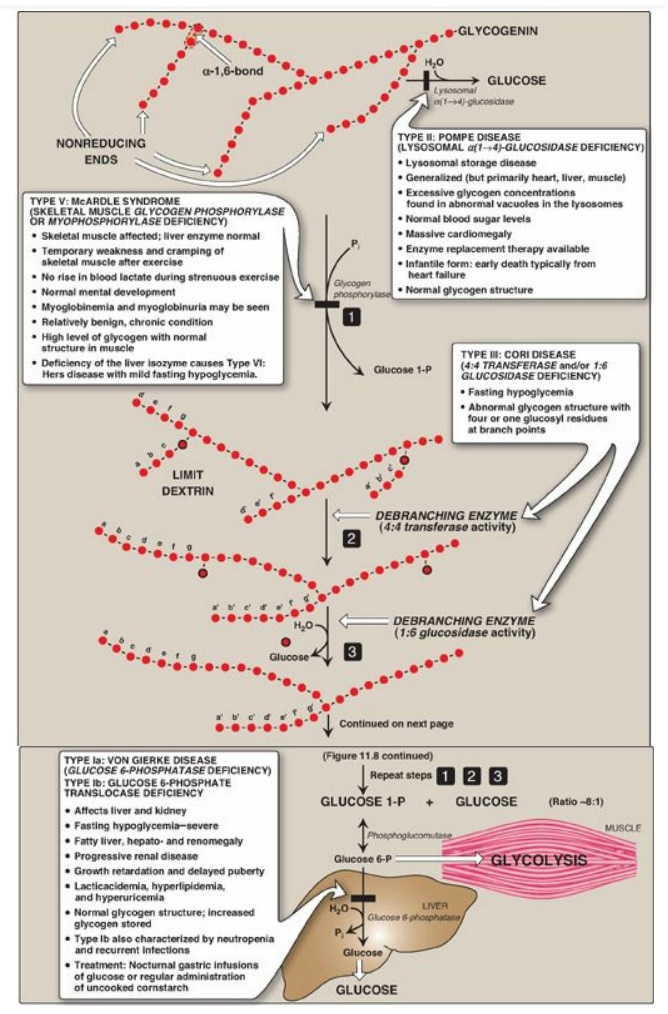
Figure 11.8 Glycogen degradation, showing some of the glycogen storage diseases (GSDs). [Note: A GSD can also be caused by defects in branching enzyme, an enzyme of synthesis, resulting in Type IV: Andersen disease and causing death in early childhood from liver cirrhosis.] Pi = inorganic phosphate; P = phosphate. Glycogen degradation, showing some of the glycogen storage diseases (GSDs).
B. Removal of branches
Branches are removed by the two enzymic activities of a single bifunctional protein, the debranching enzyme (see Figure 11.8). First, oligo-α(1→4)→α(1→4)-glucantransferase activity removes the outer three of the four glucosyl residues attached at a branch. It next transfers them to the nonreducing end of another chain, lengthening it accordingly. Thus, an α(1→4) bond is broken and an α(1→4) bond is made, and the enzyme functions as a 4:4 transferase. Next, the remaining glucose residue attached in an α(1→6) linkage is removed hydrolytically by amylo-α(1→6)-glucosidase activity, releasing free glucose. The glucosyl chain is now available again for degradation by glycogen phosphorylase until four glucosyl units in the next branch are reached.
C. Conversion of glucose 1-phosphate to glucose 6-phosphate
Glucose 1-phosphate,
produced by glycogen phosphorylase, is converted in the cytosol to glucose
6-phosphate by phosphoglucomutase (see Figure 11.6). In the liver, glucose
6-phosphate is transported into the endoplasmic reticulum (ER) by glucose
6-phosphate translocase. There it is converted to glucose by glucose
6-phosphatase (the same enzyme used in the last step of gluconeogenesis;). The
glucose then is transported from the ER to the cytosol. Hepatocytes release
glycogen-derived glucose into the blood to help maintain blood glucose levels
until the gluconeogenic pathway is actively producing glucose. [Note: In the
muscle, glucose 6-phosphate cannot be dephosphorylated and sent into the blood
because of a lack of glucose 6-phosphatase. Instead, it enters glycolysis,
providing energy needed for muscle contraction.]
D. Lysosomal degradation of glycogen
A small amount (1%–3%)
of glycogen is continuously degraded by the lysosomal enzyme,
α(1→4)-glucosidase (acid maltase). The purpose of this pathway is unknown.
However, a deficiency of this enzyme causes accumulation of glycogen in
vacuoles in the lysosomes, resulting in the serious glycogen storage disease
(GSD) Type II: Pompe disease (see Figure 11.8). [Note: Type II: Pompe disease
is the only GSD that is a lysosomal storage disease.]
Lysosomal storage diseases are genetic disorders
characterized by the accumulation of abnormal amounts of carbohydrates or
lipids primarily due to their decreased lysosomal degradation.
Related Topics
