Complexation
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Complexation
Complexes or coordination results from a donor-acceptor mechanism (donating accepting electron or rather an electron pair) or Lewis acid–base reaction (donating accepting protons).
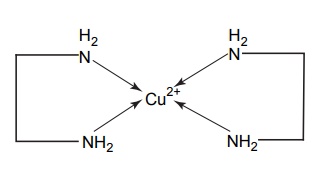
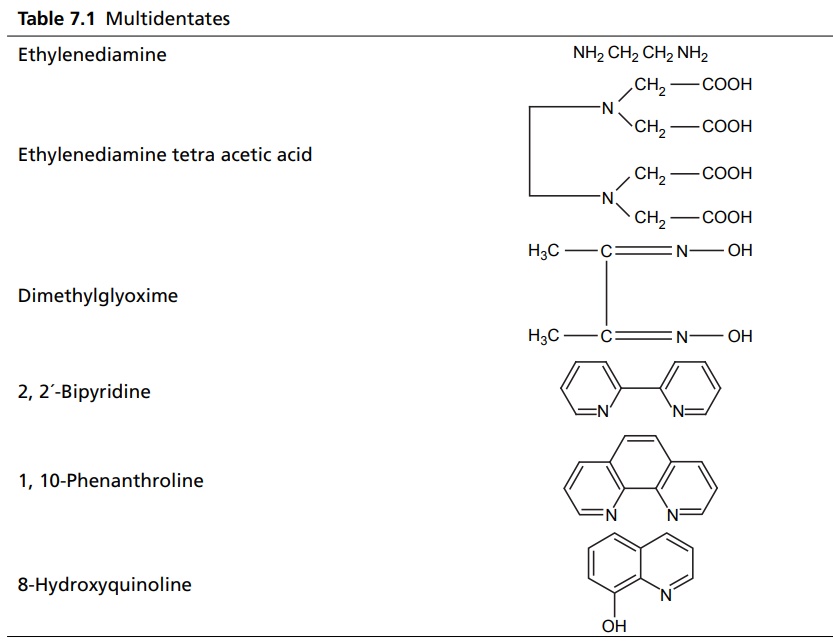
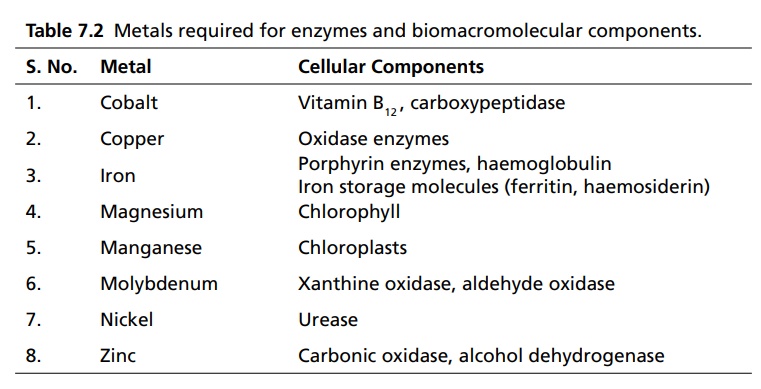
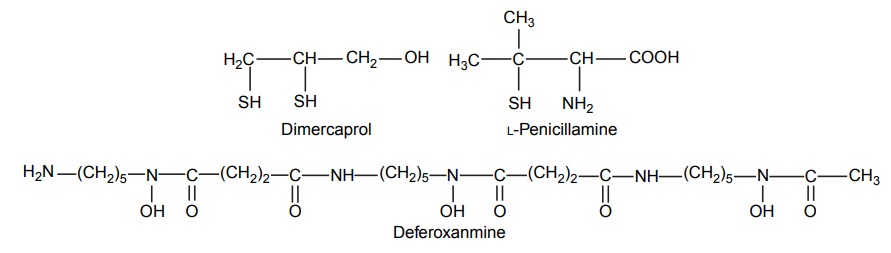
Complexation INTRODUCTION Complexes or coordination results from a donor-acceptor mechanism (donating accepting electron or rather an electron pair) or Lewis acid–base reaction (donating accepting protons). Since complex drugs cannot cross the natural membranous barriers, they reduce the rate of absorption of the drug. The compounds that are obtained by donating electrons to metal ions with the formation of ring structures are called chelates. The compounds that are capable of forming a ring structure with metal atoms are termed as ligands. Both biological molecules and medicinal agents may develop chelate structures by forming a ring structure with a metal through coordinate bonds (i.e. bonds in which electrons pairs are from the same atom). Example: Glycine forms complex with Cu 2+ The stability constant (KS) reflects the strength of the interaction, and larger the constant the greater the stability. Two coordinate covalent bonds are formed between glycine and copper in the complex. The nitrogen and oxygen atoms of glycine serve as the electron donating groups, each supplying an electron pair, whereas the cupric ion is the electron acceptor. Electron donating groups are almost always limited to O, N, and S atoms; electron acceptors include various bivalent and trivalent metals particularly those of the transition group. Stability of the metal ions in the complex: Fe3+, Hg2+ > Cu2+, Al3+ > Ni2+, Pb2+ > Co2+, Zn2+ > Fe2+, Cd2+ > Mn2+ > Mg2+ >Ca2+ A ligand, such as ammonia, that has a single basic group capable of bonding to the metal ion is a unidentate ligand. A ligand having more than one accessible basic binding site is multidentate, for example, ethylenediamine, NH2CH2CH2NH2, is a bidentate ligand form, and chelates with Cu (II). Some important multidentates are listed in Table 7.1. The number of rings formed in the chelate depends on the electron donating groups. S can form the stable four-member rings, O and N can form five and six member rings, but five member rings are generally more stable. Heavy metals are required for the following enzymes and biomacromolecular components (Table 7.2). Table 7.2 Metals required for enzymes and biomacromolecular components. Important metal binding hormones are thyroxine, insulin, histamine, epinephrine, and norepinephrine. In clinical practice, chelating agents have been used primarily as antidotes in heavy metal poisoning. In principle, any chelating agent with a sufficiently high KS value in vitro could be used as an antidote, but some limitations are there. For example, ethylene diamine tetra acetic acid (EDTA), because of its powerful chelating effect can displace toxic heavy metals, such as lead and mercury from cellular layer, but EDTA is not highly selective in its action, it tightly binds some essential metals, including calcium. To prevent excessive loss of calcium from the body during EDTA therapy, it is necessary to administer this antidote as disodium calcium salt. Iron deficiency, anaemia, may be treated using the iron complex of EDTA in the place of ferrous sulphate or ferrous gluconate. Pernicious anaemia is treated effectively using Vitamin B12, a naturally occurring cobalt complex. Deferoxamine is a highly selective antidote, which strongly chelates iron in iron poisoning. Dimercaprol (BAL) is used in the treatment of lead poisoning. L-Penicillamine is used in the treatment of copper and Wilson’s disease. Numerous antimicrobial and antineoplastic agents are believed to exert their action by means of complex formations with DNA base pairs. These drug molecules are large planar aromatic compounds, and they can be inserted between the planar base pair assemblies on the DNA double helix. This type of inserted molecular interaction is called intercalation. Intercalating drugs include ethidium, quinacrine, proflavin, daunorubicin, adriamycin, and actinomycin D. Undesirable side effects are caused by drugs that chelates with metals. A side effect of hydralazine, an antihypertensive agent, is the formation of anaemia, and this is due to chelation of the drug with iron. 8-Hydroxy quinoline and its analogues acts as antibacterial and antifungal agents by complexing with iron or copper.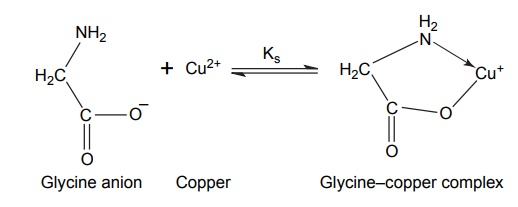
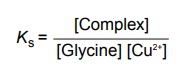
Related Topics
