Combinatorial Compound Libraries
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Combinatorial Chemistry
The origin of combinatorial chemistry lies in the use of solid supports for peptide synthesis.
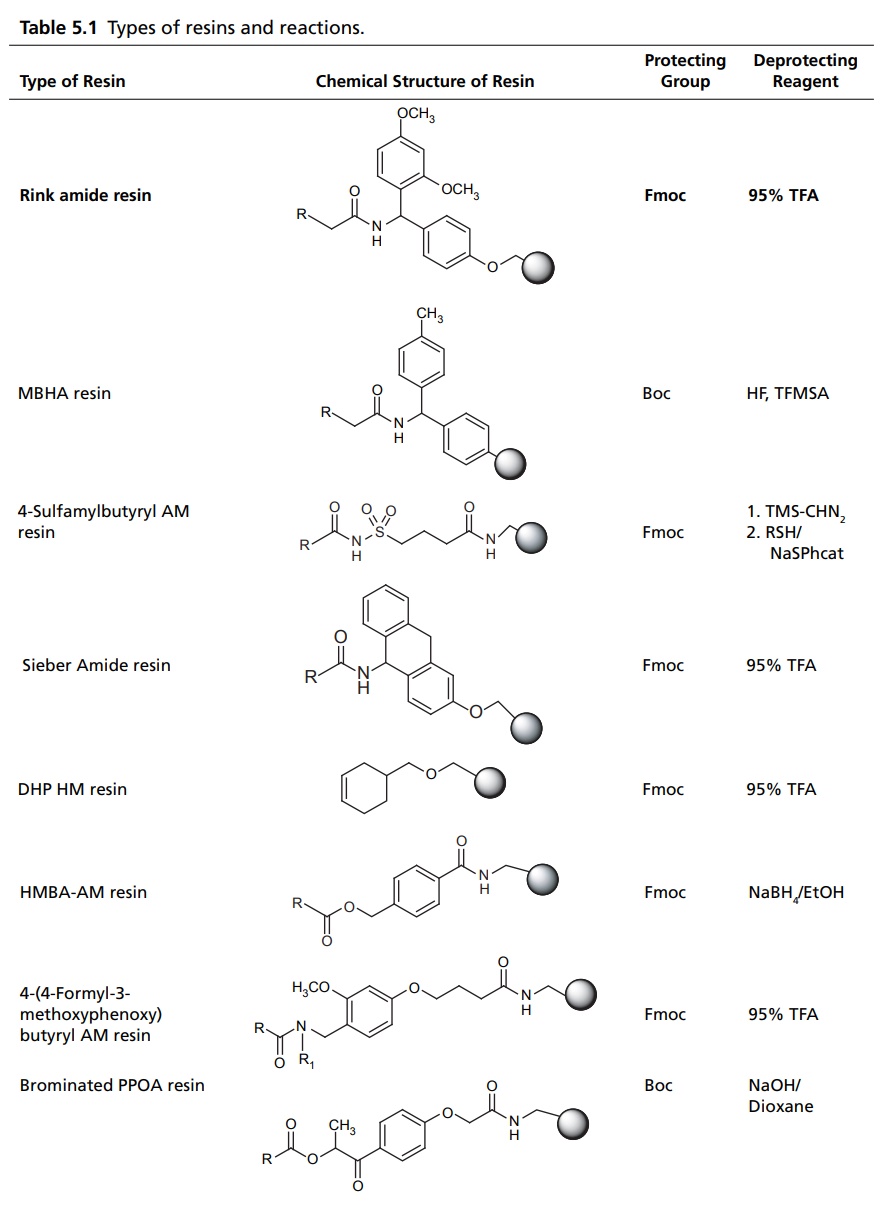
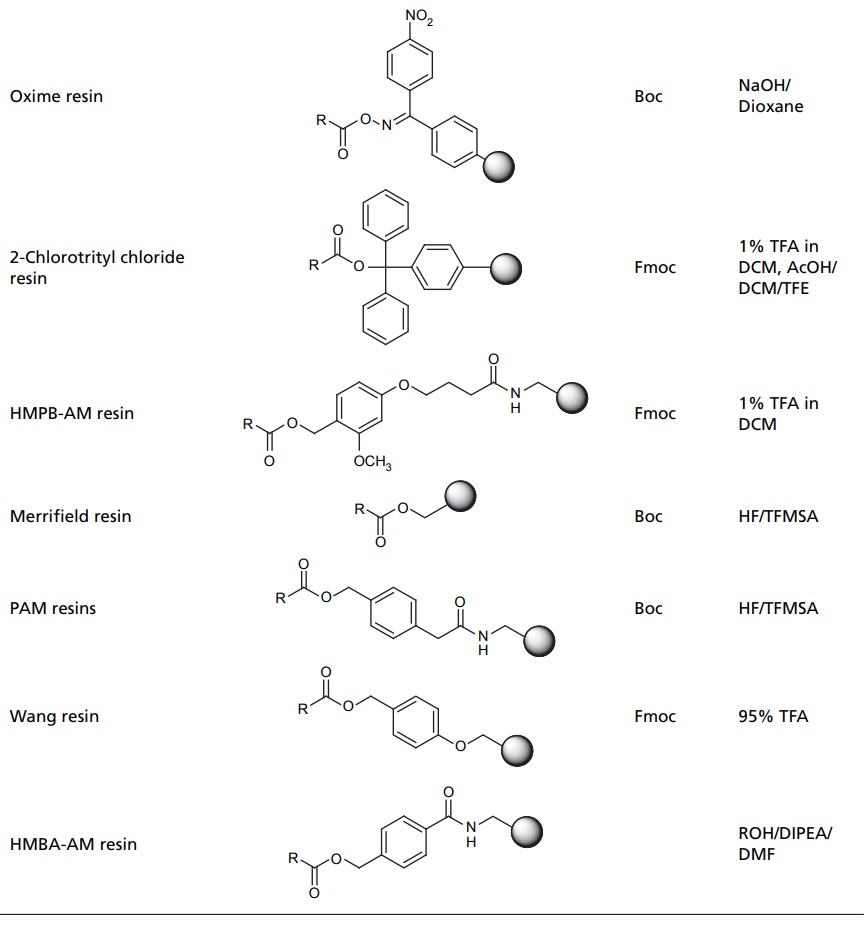
COMBINATORIAL COMPOUND LIBRARIES The origin of combinatorial chemistry lies in the use of solid supports for peptide synthesis. By coupling the growing peptide to a solid support, such as a polystyrene bead, it is possible to use excess reagents and so ensure that the reaction proceeds to completion. Any excess reagent is simply washed away. In the original applications of solid-phase chemistry to peptide synthesis the goal was generally the synthesis of a single molecular target. A key breakthrough was the recognition that this methodology could be used to generate large numbers of molecules using a scheme known as split–mix technique. This technique starts with a set of reagents (which we may also refer to as monomers), each of which is coupled to the solid support. These are then mixed together and divided into equal-sized aliquots for reaction with the second reagent. The products from this reaction are reacted with the third reagent, and so on. If the number of reagents at each step are n1, n2, n3, etc., then the total number of molecules produced is the product is n1n2n3. The size of the library, thus, increases exponentially with the number of reagents—hence the use of the term ‘combinatorial’. The original split-mix method is capable of producing extremely large libraries, but it does suffer from some drawbacks. A particular limitation is that due to the various mixing stages the identity of the product on each bead is unknown (except for the final reagent). It is important to note, however, that each bead contains just one discrete chemical entity. In the recent years, many progress has subsequently been made in the technology for automated synthesis and purification since the first reports were published. These developments have enabled many of the limitations of the early combinatorial techniques to be overcome, making automated synthesis methods a common place in both industrial and academic laboratories. In 1963, Merrifield pioneered the solid phase synthesis (SPS) work, which earned him a nobel prize. Merrifield’s SPS concept was first applied for a developed biopolymer, recently it has spread in every field where organic synthesis is involved. Nowadays, many academic laboratories and pharmaceutical companies focused on the development of the technologies and chemistry suitable for SPS. This resulted in the impressive outbreak of combinatorial chemistry, which profoundly changed the approach to new drugs, new catalyst, or new natural discovery. The utilization of solid support for the organic synthesis relies on three interconnected requirements. These are as follows: A cross-linked, insoluble polymeric material should be inert to the condition of synthesis. The linking substrate (linker) to the solid phase that permits selective cleavage of some or all the products from the solid support during synthesis for analysis of the extent of reaction (s) and ultimately to give the final product of interest. The chemical protection strategy must allow selective protection and deprotection of reactive groups. Solid-supported reagents are easily removed from reactions by filtration. Excess reagents can be used to drive reactions to completion without introducing difficulties in purification. Recycling of recovered reagents is economical, environmentally-sound, and efficient. Ease of handling is especially important when dealing with expensive or time-intensive catalysts, which can be incorporated into flow reactors and automated processes. Finely tune chemical properties by altering choice of support and its preparation. Toxic, explosive, and noxious reagents are often more safely handled when contained on solid support. Reagents on solid-support react differently, mostly more selectively, than their unbound counterparts. Some reagents may not interact well with solid support. Ability to recycle reagents on solid support is not assured. Reactions may run more slowly due to diffusional constraints. Polymeric support materials can be very expensive to prepare. Stability of the support material can be poor under harsh reaction conditions. Side reactions with the polymer support itself may occur. In solid phase synthesis, resin supports for SPS include spherical beads of lightly cross-linked gel type polystyrene (GPS) (1%–2% divinylbenzene) and poly(styrene-oxyethylene) graft copolymers, which are functionalized to allow attachment of linkers and substrate molecules. Each of these materials has advantages and disadvantages, depending on the particular application. There are several types of resins available for different type of reactions, which has been mentioned in Table 5.1.Combinatorial Synthesis on Solid Phase
ADVANTAGES AND DISADVANTAGES OF SOLID SUPPORT REAGENTS
Advantages
Disadvantages
Resins for SPS
Related Topics
