Classification of Pro-drugs
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Pro-Drugs
Depending on the constitution, lipophilicity, and method of bioactivation, pro-drugs are classified into two categories. 1. Carrier-linked pro-drugs 2. Bio-precursors
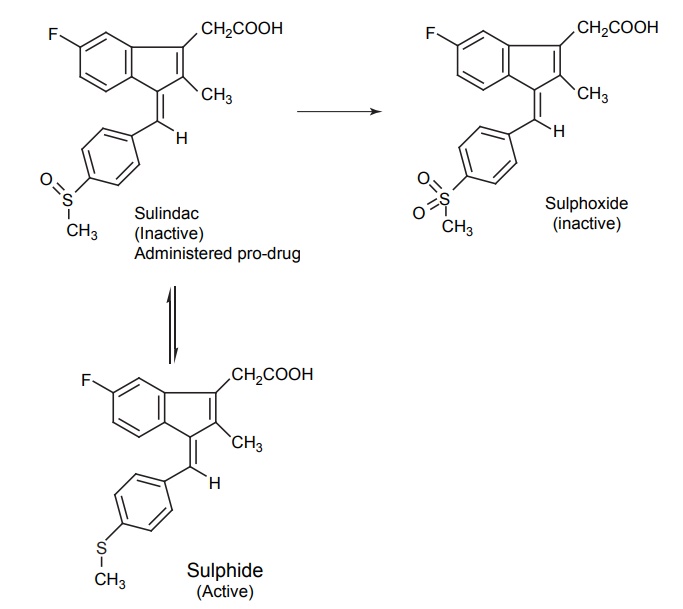
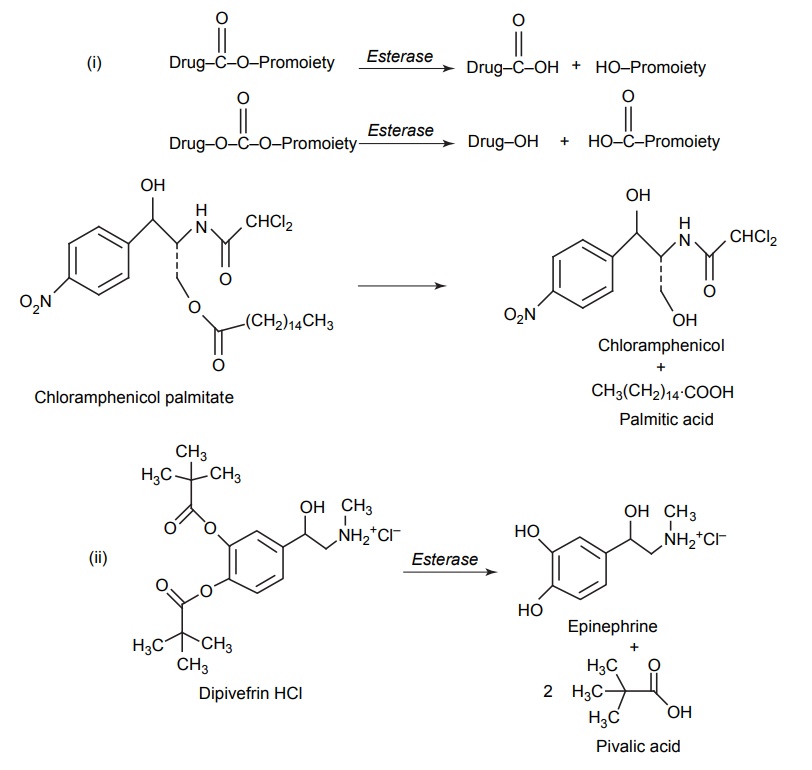
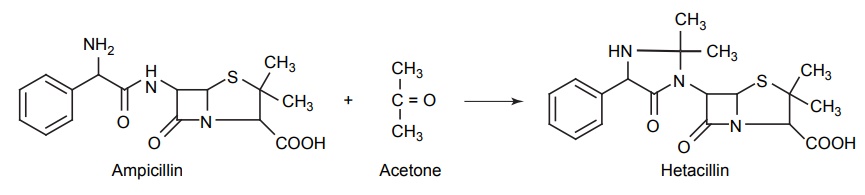
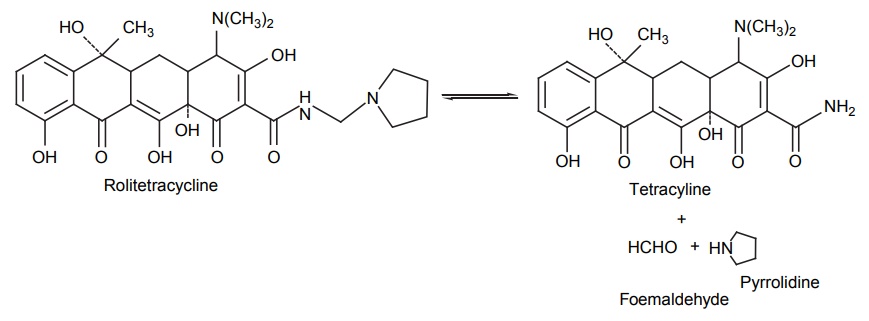
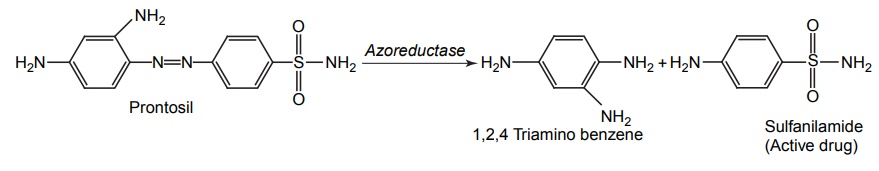
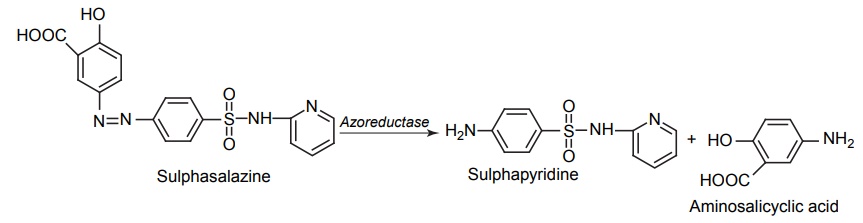
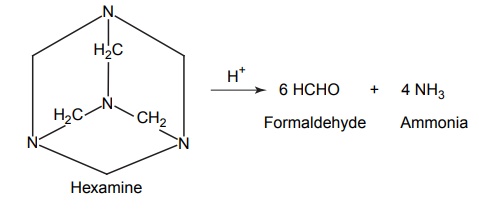
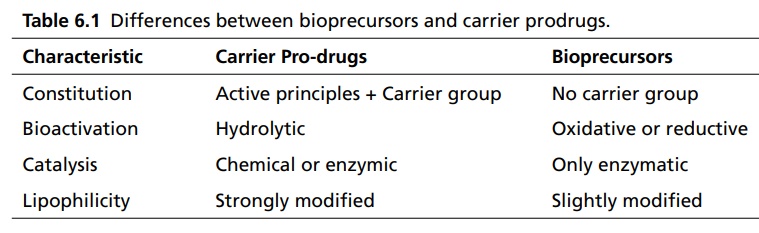
CLASSIFICATION OF PRO-DRUG Depending on the constitution, lipophilicity, and method of bioactivation, pro-drugs are classified into two categories. Carrier-linked pro-drugs Bio-precursors Carrier-linked pro-drug or simple pro-drugs: They are generally esters or amides. Carrier-linked prodrugs are the ones where the active drug is covalently linked to an inert carrier or transport moiety. Such pro-drugs modify the lipophilicity due to the attached carrier. The active drug is released by hydrolytic cleavage, either chemically or enzymatically. Examples: Bioprecursors: They are inert molecules obtained by a chemical modification of the active drugs, but do not contain a carrier. For example, nonsterodial anti-inflammatory drug, sulindac, is inactive as sulphoxide and must be reduced metabolically to active sulphide. Pro-drugs are also classified according to the functional group. They are Carboxylic acids and alcohols Amines Azo linkages Carbonyl compounds Carboxylic acid and alcohols: Pro-drugs of carboxylic acid and alcohol functionalities can be prepared by conversion to esters. The esters can be easily hydrolyzed by esterase enzymes (e.g. lipase, ester hydrolase, cholesterol esterase, acetyl cholinesterase, and carboxy peptidase) present in plasma and other tissues to give active drug. Example: Amines: Due to the high stablility and lack of amidase enzyme necessary for hydrolysis, the conversion of amines to amide as a pro-drug is not been used for most of the drugs. A more common approach adopted is to use Mannich bases as pro-drug form of amines. Hetacillin is a pro-drug form of ampicillin in which amide nitrogen and α amino functionalities have been allowed to react with acetone to give a Mannich base (imidazolidine ring system). This leads to decrease in the basicity and increase in the lipophilicity and absorption. The basic pyrrolidine nitrogen increases water solubility of the parent drug rolitetracycline. The Mannich base hydrolyzes completely and rapidly in aqueous media to give the active tetracycline. Azo linkage: Pro-drugs of amines are occasionally prepared by incorporating them in to an azo linkage. By the action of azo reductase the amino compounds are released in vivo. Prontosil drug is inactive in vitro, but it is active in vivo since it is converted to sulphanilamide by azo reductase enzymes. Sulphasalazine by the action of azo reductase releases the amino salicylic acid and sulphapyridine. The generation of anti-inflammatory salicyclic acid prior to absorption prevents the systemic absorption of the agents and enhances the concentration of it in active site. Carbonyl moiety: Conversion of carbonyl functionalities, such as aldehyde and ketone, to pro-drug have not been found wide clinical use. They are converted into derivatives in which the sp2 carbonyl carbon is converted as sp3 hybridized carbon attached to hetero-atoms. These pro-drugs are re-converted to carbonyl compound by hydrolysis. For example, hexamine releases formaldehyde in the urine (acidic PH), which acts as an antibacterial agent. The differences between bioprecursors and carrier prodrugs are given in Table 6.1.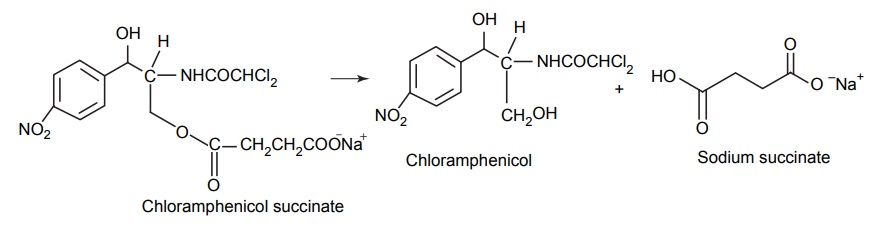
Related Topics
