Cisplatin
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Transition Metals and d-Block Meta Chemistry
CDDP, also referred to as cisplatinum or cisplatin, is a yellow powder and has found widespread use a chemotherapeutic agent. The platinum complex binds to DNA and causes cross-linking, which triggers the programmed cell death (apoptosis).
Cisplatin
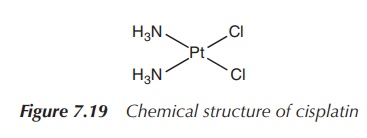
CDDP, also referred to as cisplatinum or cisplatin, is a yellow powder and has found widespread use a chemotherapeutic agent. The platinum complex binds to DNA and causes cross-linking, which triggers the programmed cell death (apoptosis). Cisplatin is specifically used as an effective therapeutic agent against ovarian, testicular, uterus, bladder and head and neck cancers (Figure 7.19).
1. Discovery
Rosenberg, a biophysicist working at Michigan State University,
discovered the anticancer activity of cisplatin in 1965 serendipitously.
Rosenberg devised an experiment to investigate the effect of electric fields on
cell division, in which he passed an alternating electric current through two
Pt electrodes immersed in a beaker containing Escherichia coli bacteria in a cell growth medium containing
ammonium and chloride ions. During the experiment, Rosenberg discovered that
the bacteria had grown in size, but not divided as was expected. On carrying
out some control experiments, it became soon clear that it was not the electric
current that caused this unusual cell growth. Rosenberg realised that a
chemical reaction had taken place in the cell medium requiring oxygen, ammonium
ions (NH4+) and chloride ions (Cl−) in
addition to a small amount of platinum, which was dissolved from the surface of
the electrodes. A mixture of platinum salts was accidentally synthesised which
contained cisplatin (cis-[Pt(II)Cl2(NH3)2]).
Rosenberg subsequently showed that only cis-[Pt(II)Cl2(NH3)2]
and not trans-[Pt(II)Cl2(NH3)2]
could prevent the growth of cancer cells in
vitro. Typically, cisplatin kills cancer cells at micromolar doses.
Nevertheless, there is a lot of work necessary in between the discovery of a cytotoxic agent and the licensing of an anticancer drug. At the time Rosenberg discovered the potential of cisplatin, only organic compounds were seen to be appropriate for medicinal use in humans and certainly a heavy metal compound was seen as being too toxic for a therapeutic approach. Rosenberg convinced research institutes such as the National Cancer Institute to carry out several tests and trials. In 1979, he finally managed to file a patent on the use of cisplatin as anticancer agent. The synthesis itself had been reported 100 years ago, and cisplatin certainly was not a novel compound anymore, which could be patented. Bristol-Myers became interested in the compound, and the FDA licensed cisplatin as an anticancer drug. This discovery led to a whole new area of drug discovery, as from this point drug development was not only limited to organic compounds anymore.
2. Mode of action
Cisplatin is a neutral, purely inorganic compound, first
synthesised in 1844, containing a platinum(II) centre and two ammonia ligands
and two chloride ligands. The ammonia ligands represent the nonleaving groups,
whereas the chloride ligands are labile and can be exchanged by nucleophiles
(Figure 7.20).
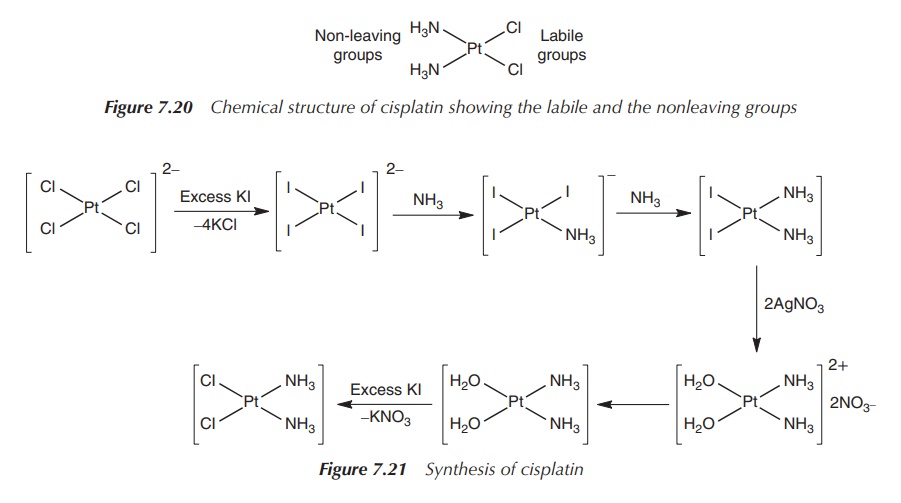
The synthesis of cisplatin starts with K2[PtCl4],
but it has undergone several improvements since it was published more than 100
years ago. The main problem is the occurrence of impurities and the synthesis
of the by-product transplatin. Nowadays, the synthetic routes are mostly based
on a method published in the 1970s by Dhara. In the initial step, K2[PtCl4]
is reacted with KI, and the platinum complex is converted into the tetraiodo
analogue. Subsequently, NH3 is added and cis-[PtI2(NH3)2] is obtained. cis-[PtI2(NH3)2]
precipitates from the solution once AgNO3 is added, and the insoluble
AgI can be filtered off. KCl is added to the solution and cisplatin is formed
as a yellow solid. The success of the synthesis relies on the strong
translabellising effect of the iodo ligands as discussed earlier (Figure 7.21).
Since the success of using cisplatin as a chemotherapeutic
agent, significant research has been undertaken to establish the exact mode of
action. Some areas still remain unclear, but it is clear that its anticancer
ability mainly stems from its ability to form adducts with DNA. It has been
generally accepted that the cytotoxic activity of cisplatin is due to the
interaction between the metal complex and the genetic DNA, which is located in
the cell nucleus .
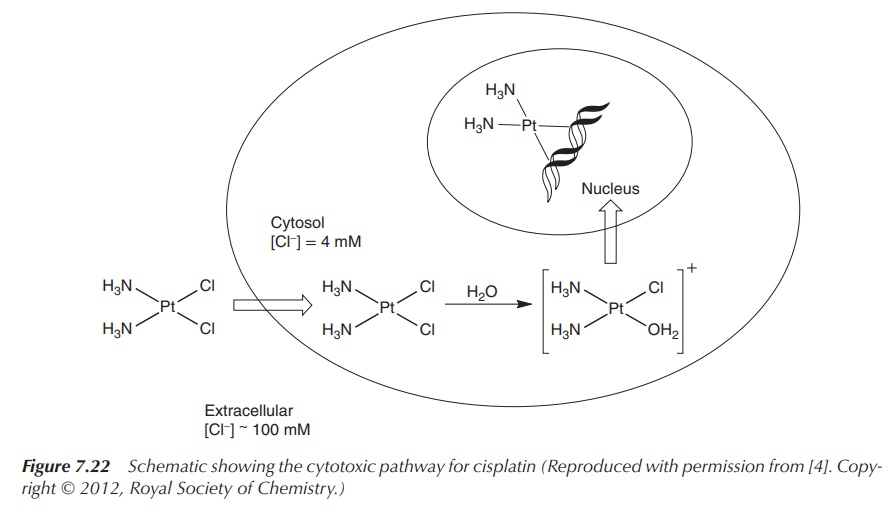
Cisplatin is administered intravenously as the
neutral complex, and transported via the blood stream to the cancer cell. The
blood stream and the extracellular fluids have a high chloride concentration (>100 mM) and therefore, the platinum complex
will not be hydrolysed. There is still much debate about the cellular uptake. It
is believed that the neutral complex enters the cancer cell by passive and/or
active transport. Apart from the passive diffusion, carrier-mediated proteins
have been identified, such as the plasma membrane copper transporter, organic
cation transporters and others (Figure
7.22).
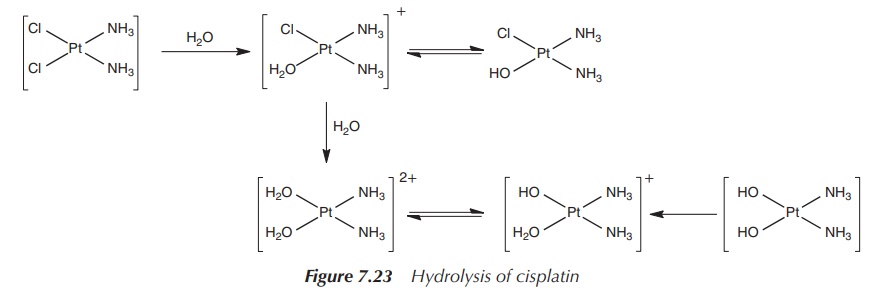
The mode of action inside the cell begins with the hydrolysis of
the platinum–chloride bonds. This hydrol-ysis is facilitated by the
significantly lower chloride concentration inside the cell (4 mM) compared to
the high chloride concentration in the blood plasma, which prevents the
hydrolysis of cisplatin during the trans-port in the blood stream. Upon
entering the cell, it is proposed that cisplatin loses its chloride ligands and
forms the mono and diaqua species. The hydrolysed species are good
electrophiles and can bind to a variety of nucleophiles in the cell, such as
nucleic acid and thiol-containing proteins (Figure 7.23).
The anticancer activity of cisplatin is based
on the interaction of the platinum complex with DNA located in the nucleus.
Interaction with the mitochondrial DNA is believed to be less important for the
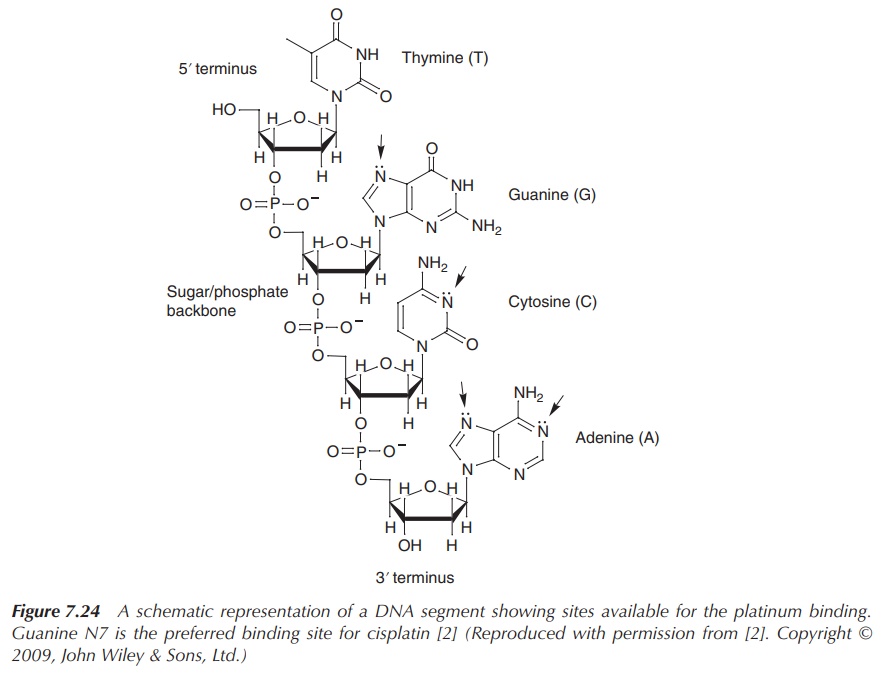
antitumour activity of cisplatin. Cisplatin binds to DNA primarily by
coordination to the nitrogen (N7) atom of guanine, whereas it also can bind (to
a lesser degree) to N7 and N1 of adenine and N3 of cytosine [2, 6] (Figure
7.24).
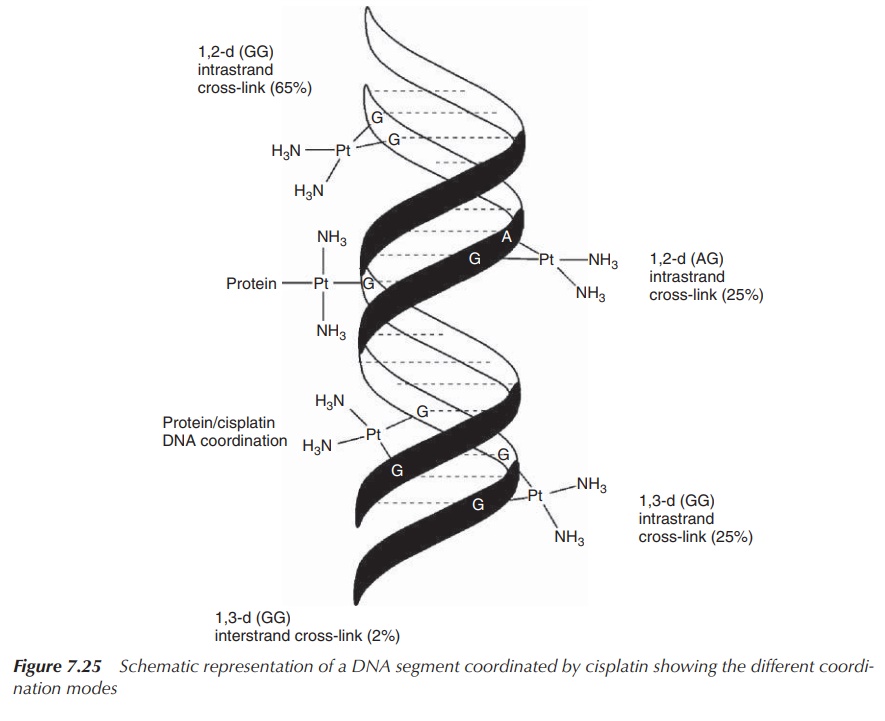
It was found that the majority of DNA–cisplatin complexes are
formed by intrastrand 1,2-(GG) cross-links, which means cisplatin coordinates
to two guanine bases within the same DNA strand. This form of DNA-adduct
formation makes up around 65% of all DNA–cisplatin complexes. Note that the
Arabic numbers refer to two adjacent nucleotides within the DNA sequence and
‘intra’ means that the cross-link occurs within the same DNA strand. Other
binding modes include intrastrand 1,2-(AG) cross-links (25%) as well as
inter-strand cross-links (between two DNA strands) and mono-functional binding
to DNA (Figure 7.25).
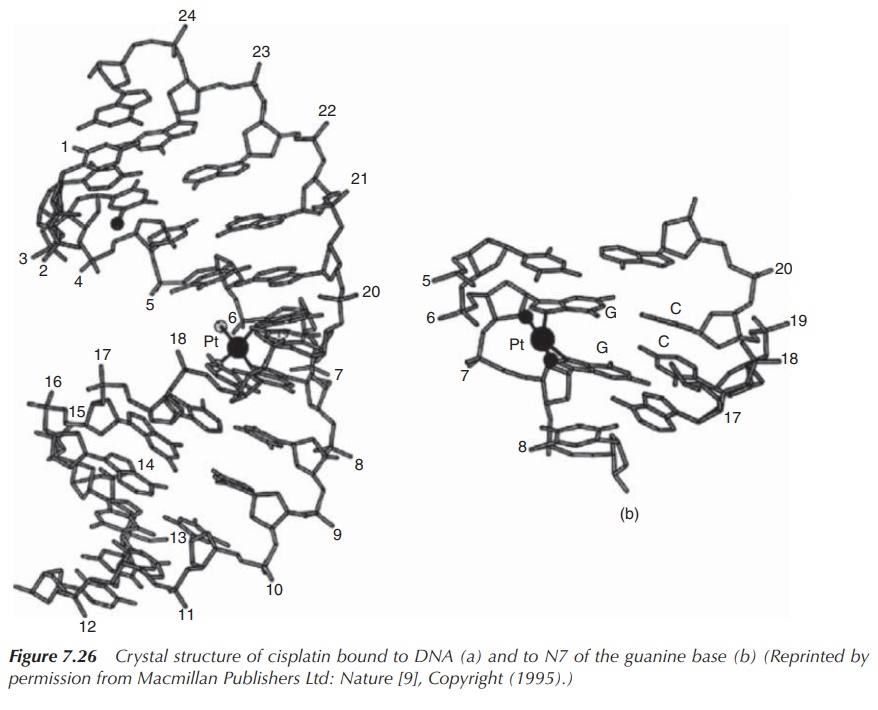
As a result of the formation of DNA–cisplatin adducts, the
secondary structure of DNA is affected. In par-ticular, the major intrastrand
cross-linking of cisplatin leads to conformational alteration in the DNA. The
plat-inum core binds to N7 of guanine bases located in the major groove and, as
a result of its square planar geome-try, forces the two bases to tilt towards
each other and away from the parallel stacked form of DNA. This leads to a
distortion of the helix axis, bending it towards the major groove. In turn,
this exposes the minor groove on the opposite site and makes it accessible for
other compounds. In essence, the formation of these adducts results in the DNA
helix to become kinked and the DNA translation to be interrupted [2, 6, 7]
(Figure 7.26).
3. Resistance and cytotoxicity
One of the major problems of chemotherapy with cisplatin is that after repeated use the cancer cells often become resistant to the treatment. Unfortunately, cisplatin has a narrow therapeutic window, which means that the difference between the therapeutically active and the toxic dose is fairly small. Therefore, it is not simply possibly to increase the dose once resistance is observed.
It has been observed that in platinum-resistant cells, less of
the metal is bound. This can be due to the cells being more effective blocking
entry to the cell, being able to move cisplatin actively out of the cell or, in
the case where the agent made its way to its target, that DNA can be repaired
and the metal complex is removed.
It has been shown that cancer cells that develop resistance to
cisplatin have a higher concentration of the sulfur-containing proteins such as
glutathione (GSH) and metallothionein (MT). Both GSH and MT can form very
stable complexes with Pt2+ via a S—Pt bond, which will reduce the
ability of the platinum complex to interact with other targets in the cell. It
is actually assumed that most of the platinum drugs bind to sulfur before it
reaches the DNA, as this interaction is the kinetically favoured process. There
is evidence that some of the Pt–thioester complexes can be broken in the
presence of DNA. Nevertheless, the Pt–thiol complexes are very stable and will
therefore inactivate the platinum drug . There is also evidence that the
Pt(II)–S adducts could be exported out of the cell via a glutathione
S-conjugate . It is interesting to note that only about 1% of the administered
cisplatin will complex with DNA .
DNA repair mechanisms are important processes within the cells and there are several different ways in which DNA can be repaired. If the DNA has been chemically modified by drugs, UV light or radicals, Nature has established a sophisticated system to check and repair the DNA and ultimately ensure the survival of the organism.
One system is called nucleotide excision repair (NER) and it uses enzymes to remove the
single-stranded DNA segment that has been modified. It also replaces this
segment by reading the opposite DNA strand. As previously discussed, the
1,2-intrastrand cross-link represents the major Pt–DNA adduct, where cisplatin
coordinates to two neighbouring guanine bases within the same DNA strand. In
recent animal experiments, it has been shown that the NER system is indeed
important to the removal of platinum from DNA. Mice with a compromised NER
system were not able to remove the platinum-coordinated segments from their DNA
in the kidney cells researched . There are also other mechanisms of resistance
under discussion, which are not further explained in this book.
4. Formulation and administration
Cisplatin is commercially available either as a powder for reconstitution or as a ready-to-use solution con-taining the drug in a saline concentration (NaCl solution) at a pH around 3.5–4.5. Under this condition, the majority of platinum is present as [PtCl2(NH3)2]. Additionally, the low pH avoids the formation of any multinuclear platinum compounds.
Cisplatin is used alone or in combination for the treatment of
bladder, lung, cervical, ovarian and testicular cancer. It is administered
intravenously and requires pre-hydration of the patient. The dose of cisplatin
depends on the body surface of the patient and ranges typically from 20 to 140
mg/m2. It can be given as a single-dose injection or as infusion
over a period of a couple of hours. Cisplatin is mainly eliminated via urine.
The highest concentrations can be found in the liver, prostate and kidneys.
Side effects include severe nausea, vomiting, ototoxicity and nephrotoxicity,
together with myelosuppression (bone marrow suppression). In particular,
nephrotoxicity is the dose-limiting factor, and close monitoring of the renal
function is required. The exact mode of damage to the kidneys is unknown, but
it accumulates in the kidneys and believed to damage the renal tubular cells
[2, 8].
5. Transplatin
Soon the question arose how transplatin (trans-diamminedichloroplatinum (II)) differs from its stereoisomer
cisplatin and how this explains the dramatic difference in efficacy. Very
early, it was seen that transplatin is not active when tested in animal models,
and therefore it was postulated that the cis geometry is crucial for the
cytotoxic activity for which two reasons where identified (Figure 7.27).
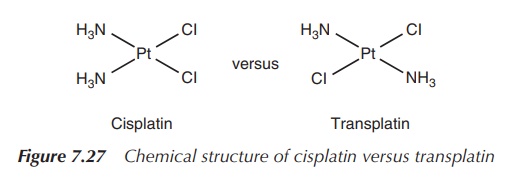
A close look at the structure of transplatin shows that the two
chloride ligands (i.e. the reactive sites) are further apart in transplatin
(4.64 Å) compared to cisplatin (3.29 Å). This affects the way the metal centre
Pt(II) can cross-link sites on DNA, which means that the 1,2 intrastrand
cross-link between the two purine bases is stereochemically hindered for the
transplatinum species (transplatin). Studies have shown that mainly the
mono-functional 1,3 adducts and inter-strand adducts are formed, which are
easily recognised by the NER repair system .
Furthermore, it has been shown that transplatin is kinetically
more reactive than cisplatin and therefore more of the compound is inactivated
before reaching its target, which contributes to the weaker activity of
transplatin. Therefore, current research focusses on the synthesis of novel
transplatin compounds in which the hydrolysis rate of the compound can be
altered .
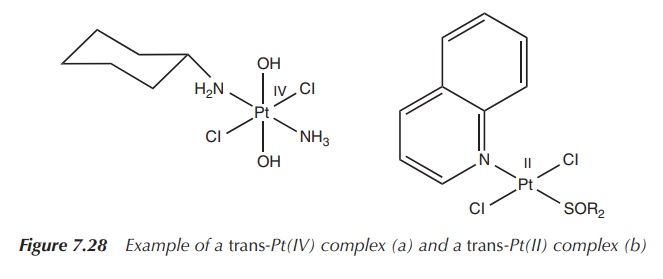
Recently, a range of transplatinum compounds were proved to have
anticancer activity. The most notable compounds are the trans-Pt(IV) complexes with the general formula trans-Pt(IV)Cl2X2LL′
or the trans-Pt(II) complexes with
the general formula trans-Pt(II)Cl2LL′.
The ligands (L and L′) are mainly imino, aromatic amine and
aliphatic amine ligands, whereas the Pt(IV) complexes contain also hydroxyl
groups. Interestingly, some of those compounds are also active on
cisplatin-resistant cell lines, thereby opening a new area of research for the
treatment of these cancer types [9, 10] (Figure 7.28).
Related Topics
