Chapter Summary, Study Questions
| Home | | Biochemistry |Chapter: Biochemistry : Globular Proteins
Hemoglobin A (HbA), the major hemoglobin (Hb) in adults, is composed of four polypeptide chains (two α chains and two β chains, α2β2) held together by noncovalent interactions.
CHAPTER SUMMARY
Hemoglobin A (HbA), the
major hemoglobin (Hb) in adults, is composed of four polypeptide chains (two α
chains and two β chains, α2β2) held together by noncovalent interactions
(Figure 3.24). The subunits occupy different relative positions in
deoxyhemoglobin compared with oxyhemoglobin. The deoxy form of Hb is called the
“T,” or taut (tense) conformation. It has a constrained structure that limits
the movement of the polypeptide chains. The T form is the low-oxygen-affinity
form of Hb. The binding of O2 to Hb causes rupture of some of the
ionic and hydrogen bonds, and movement of the dimers. This leads to a structure
called the “R,” or relaxed conformation. The R form is the high-oxygen-affinity
form of Hb. The oxygen-dissociation curve for Hb is sigmoidal in shape (in
contrast to that of myoglobin, which is hyperbolic), indicating that the
subunits cooperate in binding O2. Cooperative binding of O2
by the four subunits of Hb means that the binding of an O2 molecule
at one heme group increases the oxygen affinity of the remaining heme groups in
the same Hb molecule. Hb’s ability to bind O2 reversibly is affected
by the partial pressure of O2 (pO2) (through heme-heme
interactions), the pH of the environment, the partial pressure of CO2
(pCO2), and the availability of 2,3-bisphosphoglycerate (2,3-BPG).
For example, the release of O2 from Hb is enhanced when the pH is
lowered or the pCO2 is increased (the Bohr effect), such as in
exercising muscle, and the oxygen-dissociation curve of Hb is shifted to the
right. To cope long-term with the effects of chronic hypoxia or anemia, the
concentration of 2,3-BPG in red blood cells increases. 2,3-BPG binds to the Hb
and decreases its oxygen affinity. It therefore also shifts the
oxygen-dissociation curve to the right. Carbon monoxide (CO) binds tightly (but
reversibly) to the Hb iron, forming carboxyhemoglobin. Hemoglobinopathies are
disorders caused either by production of a structurally abnormal Hb molecule;
synthesis of insufficient quantities of normal Hb subunits, or, rarely, both
(Figure 3.25). The sickling diseases sickle cell anemia (hemoglobin S disease)
and hemoglobin SC disease as well as hemoglobin C disease and the thalassemias
are representative hemoglobinopathies that can have severe clinical
consequences.
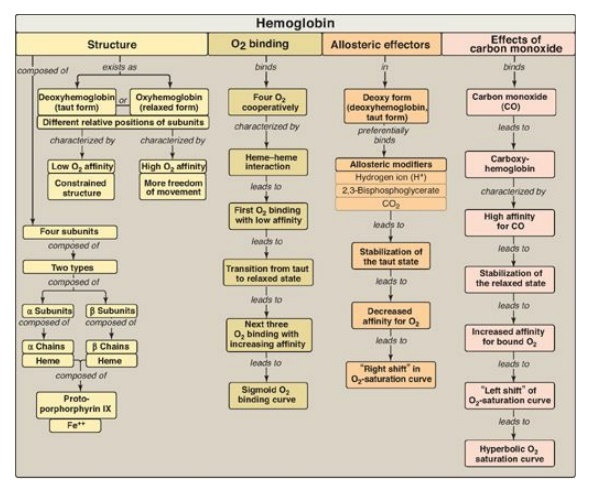
Figure 3.24 Key concept map for hemoglobin structure and function.
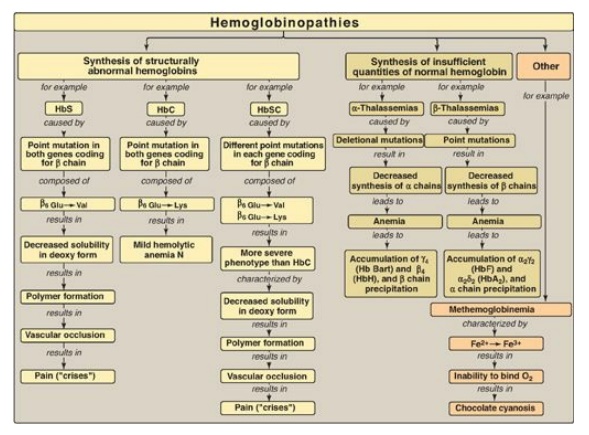
Figure 3.25 Key concept map for hemoglobinopathies. Hb = hemoglobin.
Study Questions
Choose the ONE best answer.
3.1 Which one of the following statements
concerning the hemoglobins is correct?
A. HbA is the most abundant hemoglobin in normal
adults.
B. Fetal blood has a
lower affinity for oxygen than does adult blood because HbF has an increased
affinity for 2,3-bisphosphoglycerate.
C. The globin chain
composition of HbF is α2δ2.
D. HbA1c
differs from HbA by a single, genetically determined amino acid substitution.
E. HbA2
appears early in fetal life.
Correct answer = A. HbA accounts for over 90% of the
hemoglobin in a normal adult. If HbA1c is included, the percentage
rises to approximately 97%. Because 2,3-bisphosphoglycerate (2,3-BPG) reduces
the affinity of hemoglobin for oxygen, the weaker interaction between 2,3-BPG
and HbF results in a higher oxygen affinity for HbF relative to HbA. HbF
consists of α2γ2. HbA1c is a glycosylated form
of HbA, formed nonenzymically in red cells. HbA2 is a minor
component of normal adult hemoglobin, first appearing shortly before birth and
rising to adult levels (about 2% of the total hemoglobin) by age 6 months.
3.2 Which one of the following statements
concerning the ability of acidosis to precipitate a crisis in sickle cell
anemia is correct?
A. Acidosis decreases the solubility of HbS.
B. Acidosis increases
the affinity of hemoglobin for O2.
C. Acidosis favors the
conversion of hemoglobin from the taut to the relaxed conformation.
D. Acidosis shifts the
oxygen-dissociation curve to the left.
E. Acidosis decreases
the ability of 2,3-bisphosphoglycerate to bind to hemoglobin.
Correct answer = A. HbS is significantly less soluble
in the deoxygenated form, compared with oxyhemoglobin S. A decrease in pH
(acidosis) causes the oxygen-dissociation curve to shift to the right,
indicating a decreased affinity for oxygen. This favors the formation of the deoxy,
or taut, form of hemoglobin, and can precipitate a sickle cell crisis. The
binding of 2,3-bisphosphoglycerate is increased, because it binds only to the
deoxy form of hemoglobins.
3.3 Which one of the following statements
concerning the binding of oxygen by hemoglobin is correct?
A. The Bohr effect
results in a lower affinity for oxygen at higher pH values.
B. Carbon dioxide
increases the oxygen affinity of hemoglobin by binding to the C-terminal groups
of the polypeptide chains.
C. The oxygen affinity of hemoglobin increases as
the percentage saturation increases.
D. The hemoglobin
tetramer binds four molecules of 2,3-bisphosphoglycerate.
E. Oxyhemoglobin and
deoxyhemoglobin have the same affinity for protons.
Correct answer = C. The binding of oxygen at one heme group increases the oxygen affinity of the remaining heme groups in the same molecule. A rise in pH results in increased affinity for oxygen. Carbon dioxide decreases oxygen affinity because it lowers the pH; moreover, binding of carbon dioxide to the N-termini stabilizes the taut, deoxy form. Hemoglobin binds one molecule of 2,3-bisphosphoglycerate. Deoxyhemoglobin has a greater affinity for protons and, therefore, is a weaker acid.
3.4 β-Lysine 82 in HbA is important for the binding
of 2,3-bisphosphoglycerate. In Hb Helsinki, this amino acid has been replaced
by methionine. Which of the following should be true concerning Hb Helsinki?
A. It should be stabilized
in the taut, rather than the relaxed, form.
B. It should have increased O2 affinity
and, consequently, decreased delivery of O2 to tissues.
C. Its O2-dissociation
curve should be shifted to the right relative to HbA.
D. It results in
anemia.
Correct answer = B. Substitution of lysine by
methionine decreases the ability of negatively charged phosphate groups in
2,3-bisphosphoglycerate (2,3-BPG) to bind the b subunits of hemoglobin. Because
2,3-BPG decreases the O2 affinity of hemoglobin, a reduction in
2,3-BPG should result in increased O2 affinity and decreased
delivery of O2 to tissues. The relaxed form is the
high-oxygen-affinity form of hemoglobin. Increased O2 affinity
(decreased delivery) results in a left shift in the O2-dissociation
curve. Decreased O2 delivery is compensated for by increased RBC
production.
3.5 Why is hemoglobin C disease a nonsickling
disease?
In HbC, the polar glutamate is replaced by polar lysine rather than by nonpolar valine as in HbS.
3.6 A 67-year-old man presented to the emergency
department with a 1-week history of angina and shortness of breath. He
complained that his face and extremities had a “blue color.” His medical
history included chronic stable angina treated with isosorbide dinitrate and
nitroglycerin. Blood obtained for analysis was brown colored. Which one of the
following is the most likely diagnosis?
A.
Carboxyhemoglobinemia
B. Hemoglobin SC
disease
C. Methemoglobinemia
D. Sickle cell anemia
E. β-Thalassemia
Correct answer = C. Oxidation of the heme component of
hemoglobin to the ferric (Fe3+) state forms methemoglobin. This may be caused
by the action of certain drugs such as nitrates. The methemoglobinemias are
characterized by chocolate cyanosis (a brownish blue coloration of the skin and
mucous membranes and chocolate-colored blood) as a result of the dark-colored
methemoglobin. Symptoms are related to tissue hypoxia and include anxiety,
headache, and dyspnea. In rare cases, coma and death can occur. [Note:
Benzocaine, an aromatic amine used as a topical anesthetic, is a cause of
acquired methemoglobinemia.]
3.7 What would be true about the extent of red
blood cell sickling in individuals with HbS and hereditary persistence of HbF?
Decreased. HbF reduces
HbS concentration. It also inhibits polymerization of deoxy HbS.
