Carcinogen and Mutagen Testing
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : The Wider Contribution Of Microbiology To The Pharmaceutical Sciences
A carcinogen is a substance that causes living tissues to become carcinomatous, i.e. to produce a malignant tumour. A mutagen is a chemical (or physical) agent that induces mutation in a human (or other) cell.
CARCINOGEN AND MUTAGEN TESTING
A carcinogen is a substance that causes living
tissues to become carcinomatous, i.e. to produce
a malignant tumour. A mutagen is a chemical
(or physical) agent
that induces mutation
in a human (or other)
cell.
Mutagenicity tests
are used to screen a wide variety
of chemicals for their
ability to cause
a mutation in the DNA of a cell.
Such mutations can occur at either the gene level (a point mutation) at individual chromosomes, or at the level
of a chromosome set, i.e.
a change in the number
of chromosomes (aneuploidy). Some compounds are only
mutagenic or carcinogenic after metabolism (often in the liver). This aspect
must, therefore, be considered in designing a suitable test method for such
agents.
A) Mutations At The Gene Level
Forward mutation
refers to mutation
of the natural (‘wild-type’) organism to a more stringent organism. By contrast, reverse (backward) mutation is the
return of a mutant
strain to the wild-type form,
i.e. it is a heritable change in a previously mutated gene that restores the original function of that gene.
There are two types of
reverse mutation:
•
Frame-shift.
In these mutants, the gene is altered
by the addition or deletion
of one or more bases
so that the triplex reading frame for RNA is modified;
•
Base-pair.
In these mutants,
a single base is altered
so that the triplex reading
frame is again
modified.
These principles of reverse mutation
are utilized in one
important method, the
Ames test, which is used to detect compounds that act as mutagens or carcinogens (most carcinogens are mutagens).
B) The Ames Test
The Ames
test is used
to screen a wide variety
of chemicals for potential carcinogenicity or
conversely for their
potential as cancer chemotherapeutic agents.
The test enables a large number
of compounds to be screened rapidly by examining their ability to induce mutagenesis in several specially constructed bacterial
mutants derived from
Salmonella enterica serovar Typhi. The test
strains contain mutations in the histidine operon such that they cannot
synthesize the amino acid histidine. Two additional mutations increase
further the sensitivity of the system.
The first is a defect
in their lipopolysaccharide structure such that
they are in fact deep rough mutants possessing only
2-keto-3-deoxyoctonate (KDO) linked to lipid A. This mutation
increases the permeability
of the mutants
to large hydrophobic molecules. The second mutation concerns a DNA excision repair system, which
prevents the organism
repairing its damaged DNA following exposure
to a mutagen.
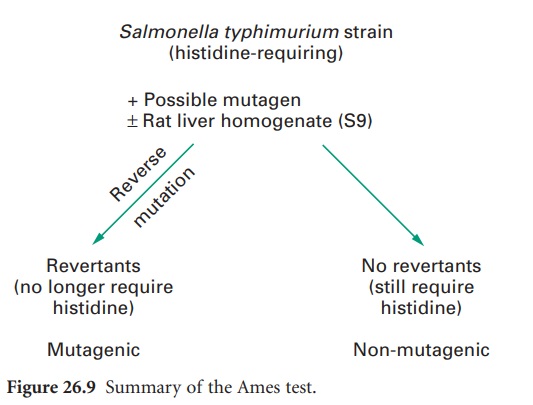
The assay
method involves
treatment of a large population of these mutant
tester strains with the test compound. Histidine-requiring mutants
are used to detect
mutagens capable
of causing base-pair substitutions (in some strains) or frame-shift mutations
(other strains). This can be carried out by incorporating both the test strain and test compound
in molten agar (at 45 °C), which is then
poured on to a minimal
glucose agar plate. Alternatively, the suspected mutagens can be
applied to the surface
of the top agar as a liquid
or as a few crystals. The medium used for the top agar contains a limited
concentration of histidine, which permits the bacteria on the plate to undergo several divisions, since for many mutagens some growth is a necessary prerequisite for mutagenesis
to occur. After incubation for 2 days at 37 °C
the number
of ‘revertant’ colonies
can be counted and compared with control plates
from which the test compound has been omitted. Each revertant colony
is assumed to be derived
from a cell that has mutated back to the wild-type and thus can
now synthesize its own
histidine: see Figure
26.9 for a summary.
A further
refinement to the Ames test permits screening of agents that require
metabolic activation before their mutagenicity or carcinogenicity is apparent. This is
achieved by incorporating into the top agar layer, along with the bacteria, homogenates of liver (commonly rat or human) whose
activating enzyme systems have been induced
by exposure to polychlorinated biphenyl
mixtures. This
test is sometimes referred to as the Salmonella/
microsome assay because the fraction of liver homogenate used, called the S9 fraction, contains
predominantly liver microsomes.
It is important to realize that
this test is flexible and is
still undergoing modification and development. Almost all the known human
carcinogens have been
tested and shown to be positive.
These include agents such as βnaphthylamine,
cigarette smoke condensates, aflatoxin B and vinyl chloride, as well as drugs used
in cancer treatment such as adriamycin, daunomycin and mitomycin C. Although the test is not perfect for the prediction of mammalian
carcinogenicity or mutagenicity and for making definitive conclusions about potential
toxicity or lack of toxicity
in humans, it nevertheless provides
useful screening information rapidly
and cheaply. The Ames test
remains an important part of a battery
of tests, the others
of which are non-microbial in nature, for detecting mutagenicity or
carcinogenicity.
Related Topics
