Bile Acids and Bile Salts
| Home | | Biochemistry |Chapter: Biochemistry : Cholesterol, Lipoprotein, and Steroid Metabolism
Bile consists of a watery mixture of organic and inorganic compounds. Phosphatidylcholine, or lecithin, and conjugated bile salts are quantitatively the most important organic components of bile.
BILE ACIDS AND BILE SALTS
Bile consists of a
watery mixture of organic and inorganic compounds. Phosphatidylcholine, or
lecithin, and conjugated bile salts are quantitatively the most important
organic components of bile. Bile can either pass directly from the liver, where
it is synthesized into the duodenum through the common bile duct, or be stored
in the gallbladder when not immediately needed for digestion.
A. Structure of the bile acids
The bile acids contain 24 carbons, with two or three hydroxyl groups and a side chain that terminates in a carboxyl group. The carboxyl group has a pKa of about 6. In the duodenum (pH approximately 6), this group will be protonated in half of the molecules (the bile acids) and deprotonated in the rest (the bile salts). The terms “bile acid” and “bile salt” are frequently used interchangeably, however. Both forms have hydroxyl groups that are α in orientation (they lie “below” the plane of the rings) and the methyl groups that are β (they lie “above” the plane of the rings). Therefore, the molecules have both a polar and a nonpolar face and can act as emulsifying agents in the intestine, helping prepare dietary triacylglycerol and other complex lipids for degradation by pancreatic digestive enzymes.
B. Synthesis of bile acids
Bile acids are
synthesized in the liver by a multistep, multiorganelle pathway in which
hydroxyl groups are inserted at specific positions on the steroid structure;
the double bond of the cholesterol B ring is reduced; and the hydrocarbon chain
is shortened by three carbons, introducing a carboxyl group at the end of the
chain. The most common resulting compounds, cholic acid (a triol) and
chenodeoxycholic acid (a diol), as shown in Figure 18.8, are called “primary”
bile acids. [Note: The rate-limiting step in bile acid synthesis is the
introduction of a hydroxyl group at carbon 7 of the steroid nucleus by
7-α-hydroxylase, an ER-associated cytochrome P450 monooxygenase found only in liver.
Expression of the enzyme is downregulated by bile acids (Figure 18.9)]
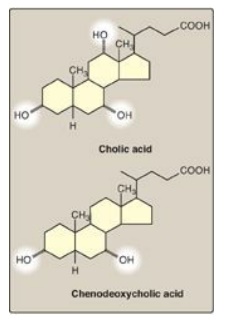
Figure 18.8 Bile acids. [Note: The ionized forms are bile salts.]
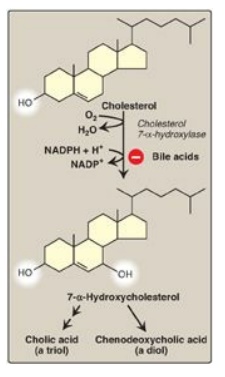
Figure 18.9 Synthesis of the bile acids, cholic acid and chenodeoxycholic acid, from cholesterol.
C. Synthesis of conjugated bile acids
Before the bile acids
leave the liver, they are conjugated to a molecule of either glycine or taurine
(an end product of cysteine metabolism) by an amide bond between the carboxyl
group of the bile acid and the amino group of the added compound. These new
structures include glycocholic and glycochenodeoxycholic acids and taurocholic
and taurochenodeoxycholic acids (Figure 18.10). The ratio of glycine to taurine
forms in the bile is approximately 3:1. Addition of glycine or taurine results
in the presence of a carboxyl group with a lower pKa (from glycine)
or a sulfonate group (from taurine), both of which are fully ionized
(negatively charged) at the alkaline pH of bile. The conjugated, ionized bile
salts are more effective detergents than the unconjugated ones because of their
enhanced amphipathic nature. Therefore, only the conjugated forms are found in
the bile. Individuals with genetic deficiencies in the conversion of
cholesterol to bile acids are treated with exogenously supplied
chenodeoxycholic acid.
Bile salts provide the only significant mechanism for cholesterol excretion, both as a metabolic product of cholesterol and as a solubilizer of cholesterol in bile.
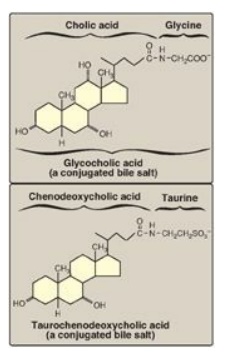
Figure 18.10 Conjugated bile salts. Note “cholic” in the names.
D. Action of intestinal flora on bile salts
Bacteria in the
intestine can deconjugate (remove glycine and taurine) bile salts. They can
also remove the hydroxyl group at carbon 7, producing “secondary” bile salts
such as deoxycholic acid from cholic acid and lithocholic acid from
chenodeoxycholic acid (Figure 18.11).
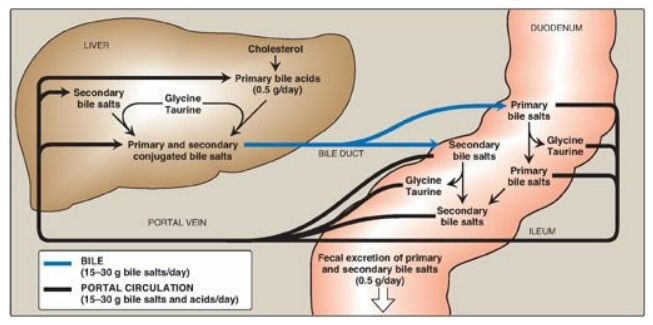
Figure 18.11 Enterohepatic circulation of bile salts. [Note: Primary forms are converted to secondary forms by dehydroxylation.]
E. Enterohepatic circulation
Bile salts secreted into the intestine are efficiently reabsorbed (greater than 95%) and reused. The liver actively secretes bile salts into the bile. In the intestine, they are reabsorbed in the terminal ileum via a Na+-bile salt cotransporter and returned to the blood via a separate transport system. [Note: Lithocolic acid is only poorly absorbed.] They are efficiently taken up from blood by the hepatocytes via an isoform of the cotransporter and reused. [Note: Albumin binds bile salts noncovalently and transports them through the blood as was seen with fatty acids.] The continuous process of secretion of bile salts into the bile, their passage through the duodenum where some are deconjugated then dehydroxylated to secondary bile salts, their uptake in the ileum, and their subsequent return to the liver as a mixture of primary and secondary forms is termed the enterohepatic circulation (see Figure 18.11). Between 15 and 30 g of bile salts are secreted from the liver into the duodenum each day, yet only about 0.5 g (less than 3%) is lost daily in the feces. Approximately 0.5 g/day is synthesized from cholesterol in the liver to replace the amount lost. Bile acid sequestrants, such as cholestyramine, bind bile salts in the gut; prevent their reabsorption; and, so, promote their excretion. They are used in the treatment of hypercholesterolemia because the removal of bile salts relieves the inhibition on bile acid synthesis in the liver, thereby diverting additional cholesterol into that pathway. [Note: Dietary fiber also binds bile salts and increases their excretion.]
F. Bile salt deficiency: cholelithiasis
The movement of
cholesterol from the liver into the bile must be accompanied by the simultaneous
secretion of phospholipid and bile salts. If this dual process is disrupted and
more cholesterol is present than can be solubilized by the bile salts and
phosphatidylcholine present, the cholesterol may precipitate in the
gallbladder, leading to cholesterol gallstone disease, or cholelithiasis
(Figure 18.12). This disorder is typically caused by a decrease of bile acids
in the bile. Cholelithiasis also may result from increased secretion of
cholesterol into bile, as seen with the use of fibrates (for example,
gemfibrozil) to reduce cholesterol (and triacylglycerol) in the blood.
Laparoscopic cholecystectomy (surgical removal of the gallbladder through a
small incision) is currently the treatment of choice. However, for patients who
are unable to undergo surgery, oral administration of chenodeoxycholic acid to
supplement the body’s supply of bile acids results in a gradual (months to
years) dissolution of the gallstones. [Note: Cholesterol stones account for
over 85% of cases of cholelithiasis, with bilirubin and mixed stones accounting
for the rest].
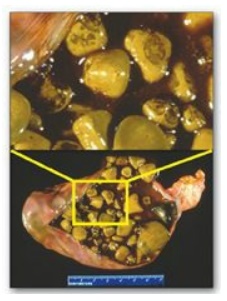
Figure 18.12 Gallbladder with
gallstones.
Related Topics
