Basic Structure of Antibody Molecule
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Immunology
The basic monomer structure can be considered the same for all the different classes of antibody (see below) even though some may form higher order structures, e.g. IgM is a pentamer made up of five antibody monomer units.
Basic Structure Of Antibody Molecule
Figure 9.4 shows an antibody monomer with
a four polypeptide subunit structure, where the subunits are linked through di-sulphide
bonding. The basic monomer structure can be considered the same for all the
different classes of antibody (see below) even though some may form higher order
structures, e.g. IgM is a pentamer made up of five antibody monomer units.
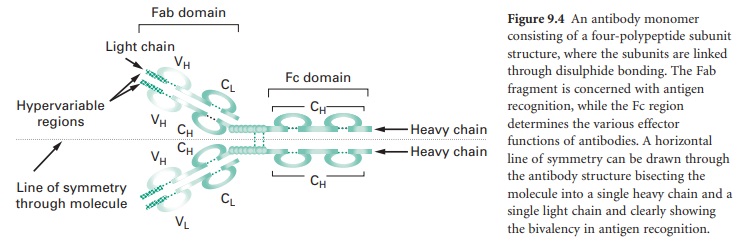
The subunits of the antibody monomer
comprise two identical ‘heavy’ polypeptide chains and two identical ‘light’
polypeptide chains, with each of these containing a ‘constant’ region and a
‘variable’ region. The light chain variable regions (VL) and the heavy chain
variable regions (VH) are the parts of the antibody molecule involved in
antigen recognition. Specifically, antibodies produced by different
B-lymphocytes or plasma cells will have variable regions possessing different
amino acid sequences leading to differences in antibody variable region surface
conformation. At the extreme tips of the variable regions are hypervariable
domains that serve the specific antigen recognition function discriminating
between, for example, diphtheria toxin and tetanus toxin. The structural
differences in the variable and hypervariable domains enable different antibodies
to recognize different structural epitopes; this meets the needs of the immune
system to combat a large and diverse range of antigens.
A horizontal line of symmetry can be drawn
through the antibody structure in Figure 9.4, bisecting the molecule into two
equivalent halves each containing a single heavy chain and a single light chain
and clearly showing the antibody monomer to possess bivalency in its ability to
interact with antigen, i.e. each antibody monomer can bind two epitopes,
although the epitopes bound by a single antibody must be identical. The antigen
recognition domain of an antibody monomer is termed the Fab domain. The
structure of the constant region of the heavy chain (CH) does not influence the
antigen recognition function of the molecule but defines the different classes
of antibody that are produced and hence the effector functions arising from
antigen–antibody interaction; this heavy chain constant region is termed the Fc
domain.
An analogy that may assist visualization
of the function of an antibody molecule is one that views it as a hand (Fab
domain) attached to the arm (Fc domain) (Figure 9.4). The palm of the hand
(variable region) can take up different shapes to allow the fingertips
(hypervariable regions) to gain a very precise interaction with an object
(antigen). At the wrist (hinge region) the hand is highly flexible relative to
the arm (Fc domain) to allow the hand and fingertips (Fab domain) maximum
flexibility to orientate an interaction with objects (antigen). The structure of
the arm (Fc domain) does not influence interaction with an object (antigen).
Once the object (antigen) has interacted with the fingertips (hypervariable
regions) of the hand then the arm (Fc domain) can mediate a variety of effector
functions.
A B-lymphocyte and plasma cell can produce
different classes of antibody depending on the stage of immune activation and
on the intercellular signals that the B-lymphocyte and plasma cell receive from
other effector cells within the immune system. As stated above, the class of
antibody is determined by the structure of the Fc domain and the different
classes of antibodies possess different effector functions. The basic classes
of antibodies are: IgM (heavy chain constant region defined as µ); IgA (heavy
chain constant region defined as α); IgD (heavy chain constant region defined
as δ); IgG (heavy chain constant region defined as γ) and IgE (heavy chain
constant region defined as ε). The different classes of antibody can be
remembered using the acronym MADGE. In addition to the heavy chain constant
region classes, there are two light chain constant region classes, κ and λ;
however, these do not mediate different antibody effector functions.
Each B-lymphocyte and the plasma cell that
derives from it is capable of producing all the different antibody classes.
However, all the antibody classes produced by a single B-lymphocyte and its
derived plasma cell will recognize only a single epitope, i.e. only a single
specific set of chemical features within a sequence or pattern of amino acid
residues. In other words, all antibodies produced by a single B-lymphocyte, and
its derived plasma cell, possess the same Fab domain recognizing the same
antigenic determinant but clearly may possess different Fc domains capable of
mediating different effector functions. Thus the same epitope can stimulate
various different forms mediated via the IgM, IgA, IgD, IgG, IgE classes of
humoral immune attack.
Within the antibody pool it is estimated
that there are approximately 109 different epitope recognition specificities,
sufficient to cover the range of pathogens likely to be encountered in life.
This enormous diversity in antigen recognition is due to the amino acid
sequence diversity in the variable and hypervariable domains of the antibody molecule.
However, this large diversity cannot result from the presence of an equivalent
number of separate protein-coding genes; the human genome project has estimated
there to be only approximately 30 000 protein-coding genes. Rather, the clonal
diversity in antigen recognition is due in the main to a process termed gene
rearrangement, which occurs in each B-lymphocyte during maturation in the bone
marrow. For example, the DNA coding for a single heavy chain molecule will
result from the splicing together of genes from four separate regions termed a
variable region gene (V), a diversity region gene (D), a joining region gene
(J) and a constant region gene (C). There are approximately 100 V genes, 25 D
genes and 50 J genes. Gene rearrangement will allow combinatorial freedom for
any V, D and J genes to splice together, providing a large number of VDJ
combined gene product permutations and hence diversity in antigen recognition.
Inaccurate splicing together of the regional genes at the V–D and D–J junctions
further increases diversity, as does the process of random nucleotide
insertion. The C genes dictate the different classes of antibody and not the
antigen recognition specificity. An additional process which occurs in a
B-lymphocyte memory cell population while it resides within the lymphoid tissue
is that of somatic mutation, in which only very slight changes in antibody Fab
domains occur through single base mutations. Sometimes these mutations prove
advantageous by increasing the affinity of an antibody to the same original
epitope. Under these circumstances the antibody clone with the highest binding
affinity to the original target epitope will proliferate and dominate. The
light chain gene also has V, J and C regions and the V and J genes undergo a
similar rearrangement to that described for the heavy chain, and hence further
add to diversity. The heavy chain and light chain polypeptides are joined
together via disulphide bond formation following protein synthesis of the
individual heavy and light chains. In summary, all antibodies produced by a
single B-lymphocyte and its derived plasma cell are ‘programmed’ to recognize
only a single antigen recognition feature determined by the recombination
pattern of the V, D and J genes (heavy chain) and the V and J genes (light
chain). The class of antibody is determined by further excisions within the DNA
to allow the same VDJ gene combination to lie next to a different C gene, which
codes for the structure of the antibody constant region and therefore
determines antibody class. The five C gene classes are m, a, d, g and e,
although various subclasses also exist. Antibody class switching is not a
random process but one that is regulated by helper T-lymphocyte cytokine
secretions.
Related Topics
