Application of Pro-drugs
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Pro-Drugs
The aim of pro-drug development is, in most cases, to solve specific pharmaceutic or pharmacological and pharmacokinetic problems.
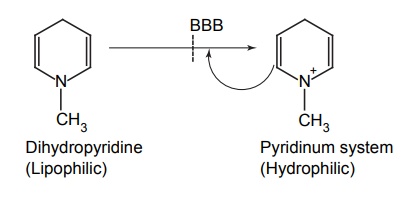
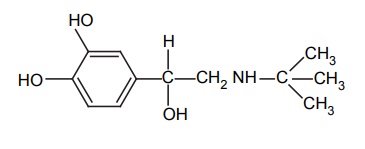
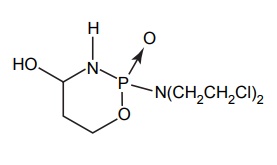
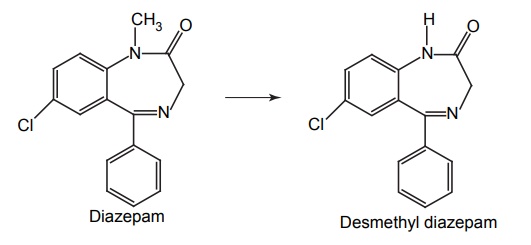
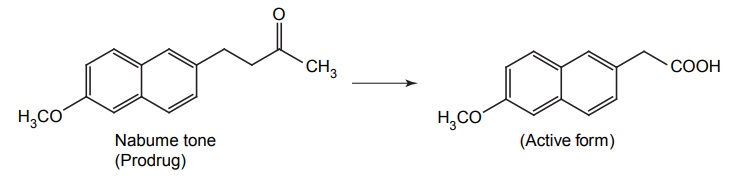
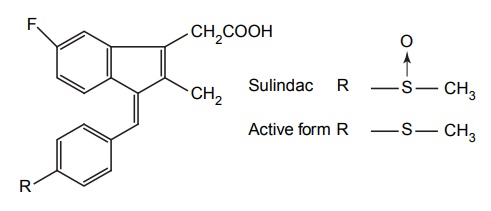
APPLICATIONS OF PRO-DRUG The aim of pro-drug development is, in most cases, to solve specific pharmaceutic or pharmacological and pharmacokinetic problems. The main objectives of pro-drug are as follows: Improvement of taste. Improvement of odour. Enhancement of bioavailability. Improvement of stability and solubility properties. Decreased toxicity and adverse reactions. Increased site specificity. Increased duration of pharmacological actions. Drug absorption, distribution, metabolism, and excretion affect pharmacokinectis. One of the reasons for poor patient compliance particularly in case of children is the bitterness, acidity, or causticity of the drug. Two approaches are adopted to overcome the bad taste of drug. The first is reduction of drug solubility in saliva and the other is to lower the affinity of drug towards taste receptors, thus, masking the bitterness. Some examples of drugs with improved taste are given in Table 6.2. The odour of a compound depends on its vapour pressure; a liquid with high vapour will have a strong odour. For example, ethyl mercaptan is a foul smelling liquid used in the treatment of leprosy. This is converted to phthalate ester, a diethyl dithioisophthalate that has higher boiling point and is odourless. Due to the presence of an amino group in the side chain, Ampicillin possesses low lipophilicity and is only 30%–40% absorbed when taken by oral route. Altering the polarity of this antibiotic, by esterifying the free carboxyl group results in compounds that are completely absorbed, that is, with greater bio-availability than the parent ampicillin. Stability: To improve their stability, prodrug approach is a good technique. Several drugs may decompose in their shelf life or in the gastro intestinal tract (GIT) when used orally. An antineoplastic drug, Azacytidine, hydrolyse readily in acidic pH, but the bisulphite prodrug of it is more stable Solubility: Hydrophilic or water-soluble drugs are needed when parenteral or ophthalmic formulation of such agents is desired. Drugs with hydroxyl functional group can be converted to their hydrophilic form through the use of half ester such as hemi-glutarate or hemi-phthalates, the other half of this acid carries sodium, potassium, or amine salts, and renders the moiety more soluble. Carboxylic acids and phenols are sometimes too toxic to be employed as such in clinical practice. Ester prodrugs of the acidic nonsteroidal anti-inflammatory drugs are devoid of gastric ulcerogenic activity and is considered as one of the responsible factors for the adverse reaction of these drugs. Many pro-drugs could be so prepared that they will be delivered to a specific site, thus reducing the toxicity to other organs. The dihydropyridine/pyridinium redox chemical delivery system is very useful for the brain. The pro-drug di-p-toluate ester of N-tbutyl noradrenaline provides a longer duration of bronchodilator activity than the parent drug. The pro-drug is preferentially distributed into the lung tissues rather than into the plasma or the heart, so that the bronchodilator effect is exerted. Bio-precursor pro-drug does not contain a carrier or a promoiety, but rather contains a latent functionality that is metabolically or chemically transformed into active drug molecule. The types of activation involves phase I, such as oxidation, reduction, phosphorylation, or chemical activation. Bio-activation: Hydroxylation of cyclophosphamide followed by the metabolite decomposition converts the pro-drug into the cytotoxic phosphoxamide mustard. N-dealkylation: Many drugs are transformed into their active metabolite form by N-dealkylation Oxidation: The prodrug nabumetone in which the formyl group formed is oxidized into a carboxylate group and generating the active drug. Reduction: The nonsteroidal anti-inflammatory drug sulindac is reduced in vivo to the active form.Improvement of Taste
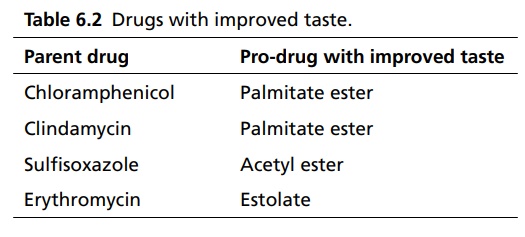
Improvement of Odour
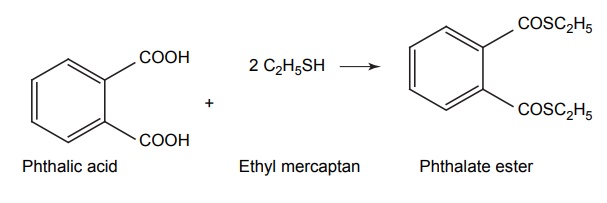
Enhancement of Bio-Availability (Lipophilicity)
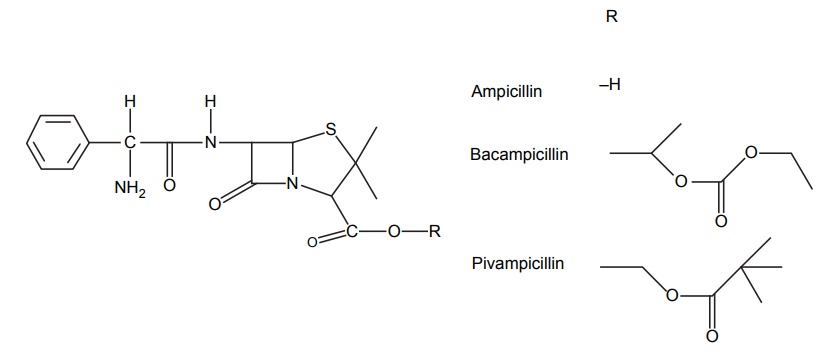
Improvement of Stability and Solubility
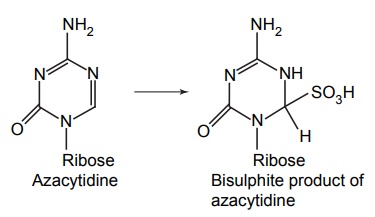

Decreased Toxicity and Adverse Reactions
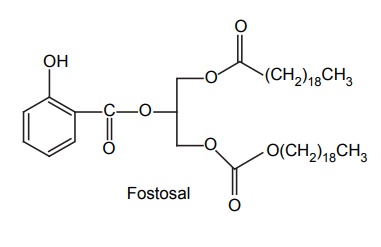
Site-Specific Drug Delivery
Increased duration of action
BIO-PRECURSOR PRODRUG
Related Topics
