Analytical Methods For Microbial Assays
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Microbiological (Microbial) Assays: Antibiotics-Vitamins- Amino Acids
There are several sophisticated analytical methods that are used most abundantly for the precise quantitative methods microbial assays.
ANALYTICAL
METHODS FOR MICROBIAL ASSAYS
There are
several sophisticated analytical methods that are used most abundantly for the
precise quantitative methods microbial assays,
such as :
(1) High
Performance Liquid Chromatography (HPLC),
(2) Reverse-Phase
Chromatography (RPC), and
(3) Ion–Pair
(or Paired-Ion) Chromatography,
These three chromatographic techniques shall
now be discussed briefly in the sections that follows :
1. High Performance Liquid Chromatography [HPLC]
Preamble : Giddings* (1964) rightly
predicted that the careful and meticulous application of relatively ‘small
particulate matter’ under the influence of excessively enhanced flow
pressure could definitely improve upon the performance of ‘Liquid Chromatography’ significantly ; and ultimately one could
easily, accomplish an appreciably high number of ‘theoretical plate numbers’. Towards the later half of 1960s
world’s two eminent scientists, Horvath and Lipsky at Yale University (USA),
came forward with the first ever HPLC,
and named it as ‘high pressure liquid
chromatography’. Neverthe-less, the early 1970s the world witnessed the
ever glorious technological supremacy by producing and using very small silanized silica particles
that gainfully permitted the usage of small-volume
longer columns absolutely urgent
and necessary to yield the much desired
high-resolution performance. In fact,
the latest HPLC is, therefore,
commonly known as the ‘high-performance
liquid chromatogra-phy’ across the globe.
Principles : The particle size of the stationary phase material predominantly
plays an ex-tremely vital and crucial role in HPLC. In actual practice, high-efficiency-stationary
phase materials have been duly researched and developed exclusively for
HPLC with progressively smaller partricle size invariably known as ‘microparticulate column packings’.
These silica particles are mostly
uni-form, porous, with spherical or irregular shape, and with diameter ranging
betwene 3.5 to 10 μm.
The bonded-phase supports normally overcome
a good number of cumbersome and nagging serious problems that are invariably
encountered with the adsorbed-liquid phases. Thus, the molecules containing the
stationary phase i.e., the surfaces of the silica particles are covalently bonded upon a silica-based
support particle.
Example : Siloxanes are duly
formed by heating the silica particles in diluted acid for 24–48 hrs. in order
to give rise to the formation of the reactive silonal moiety as depicted below :
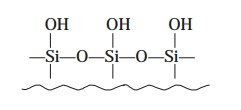
which is
subsequently treated with an organochlorosilane
:
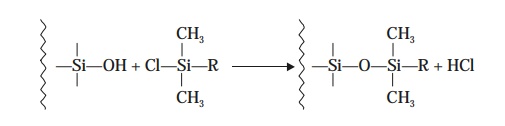
When such
microparticulate-bonded-phases are
compactly packed into a column, the tiny size of these particles affords a
substantial resistance to the ensuing solvent flow ; and, therefore, the mobile
phase has got to be pumped via the
column at a flow rate ranging between 1
to 5 cm3 . min– 1.
Advantages of HPLC : The advantages of HPLC are as stated below
:
(1) Highly
efficient, selective, and broad applicability.
(2) Only
small quantum of sample required.
(3) Ordinarily
non-destructive of sample.
(4) Rapidly
amineable and adaptable to ‘Quantitative
Analyses’.
(5) Invariably
provide accurate, precise, and reproducible results.
HPLC-Equipments : Modern
HPLC essentially comprises of seven vital
components, namely : (a) solvent reservoir and degassing
system, (b) pressure, flow, and
temperature, (c) pumps and sample
injection system, (d) columns, (e) detectors, (f) strip-chart recorder, and (g)
data-handling device and PC-based control.
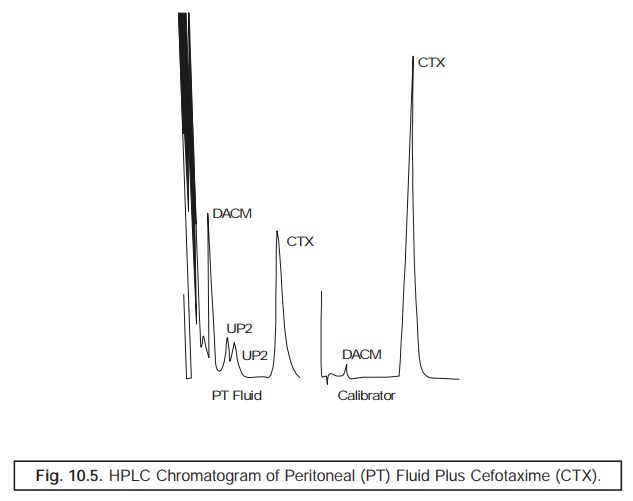
Fig. 10.5
represents the HPLC chromatogram of peritoneal
(PT) fluid from a subject having an impaired renal function to whom ‘Cefotaxime’, an antibiotic has been administered intraperitoneally. Cefotaxime (CTX) gets metabolized to
microbioligically ‘active’ and ‘inactive’ metabolites.
PT Fluid
: Peritoneal Fluid
DACM :
Desacetyl Cefotaxime (Active)
CTX :
Cefotaxime
UP1 and
UP2 : Two microbiologically inactive
metabolites
2. Reverse-Phase Chromatography [RPC]
The Reverse-Phase Chromatography (RPC) or Reversed-Phase HPLC (RP-HPLC) is
invariably employed for the separation of organic compounds.
In RPC, specifically a relatively nonpolar stationary phase is employed
along with such polar mobile phase as :
·
methanol, acetonitrile, tetrahydrofuran, water, or
·
mixture of organic solvents and water.
Organic Solvent—the organic solvent is usally
termed as the ‘modifier’ e.g., acetonitrile.
Water—Water content is mostly varied
according to the required polarity.
Methanol—It is used for acidic compounds.
Acetonitrile—It is employed for basic compounds.
Tetrahydrofuran (THF)—It is
usually used for those compounds having
large dipoles comparatively.
In fact,
most of these solvents do have low
viscosity and are UV-transparent.
Bonded Phases—The abundantly used bonded phases
are :
·
n-Octyldecyl
(i.e., C-18 chain),
·
n-Decyl (i.e., C-8 chain), and
·
Phenyl Moieties
Polar-Reversed Phase Columns— The
polar-reversed phase columns essentially are polyethylene glycol (PEG) which contain either moieties that
interact with polar analytes e.g., phe-nolic compounds, multiaromatic ring systems, and hydroxyl-containing compounds.
3. Ion-Pair (or Paired-Ion) Chromatography
Importantly,
perhaps the most valuable of the secondary
equilibria variants usually encoun-tered in the ‘pharmaceutical analysis’ being the ion-pair formation, that may be adequately expressed for a reversed-phase LLC-System as :
AM+
+ BM– ↔ ABS
where,
A+
= Might be a ‘drug cation’,
B–
= An ‘ion-pairing anion’ added to
the mobile phase
AB =
Ion-pair generated.
It has been duly observed that the
ion-pair AB thus formed is capable of partitioning very much into the ensuing
stationary phase. However, in many instances the ions A+ and B–
fail to do so by virtue of the fact that their ultimate polarity gain entry
into the stationary-phase gradually thereby the evolved chromatographic
resolution is controlled exclusively by the so
called ion-pairing
It is, however, pertinent to state
here that one may invariably come across a host of ‘drug sub-stances’ that are either acidic or basic in character ; and, therefore,
they may be duly rendered into ionic by
carefully regulating the pH of the ensuing mobile
phase. In short, ion-pair
chromatography pos-sesses an enormous applicability in the separation of drug substances.
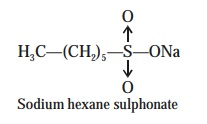
Examples : A few-typical examples pertaining to the ion-pair chromatography are as
(1) Separation of Niacin,
Niacinamide, Pyridoxine, Thiamine and Riboflamin. The admix-ture of five vitamins can be separated effectively
by making use of the sodium hexanesulphonate as the ion-pairing agent, on a C—18 col-umn i.e., ODS-column
(2) Antihistamines and decongestants may be
separated efficaciously on phenyl column.
Related Topics
![Natural Resistance and Nonspecific Defense Mechanisms [or Defensive Mechanisms of Body]](https://www.pharmacy180.com/media/article/small/empty.jpg)