What are metallocenes?
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Organometallic Chemistry
Metallocenes are a special class of the so-called sandwich complexes in which the central metal lies between two cyclopentadienyl (Cp−) ligands. Their chemical formula is typically of the type ( η5-Cp)2M, which means each Cp− ligand forms five bonds with the metal centre .
What
are metallocenes?
Metallocenes are a special class of the
so-called sandwich complexes in which the central metal lies between two
cyclopentadienyl (Cp−) ligands. Their chemical formula is typically
of the type ( η5-Cp)2M, which means each Cp−
ligand forms five bonds with the metal centre (Figure 8.4).
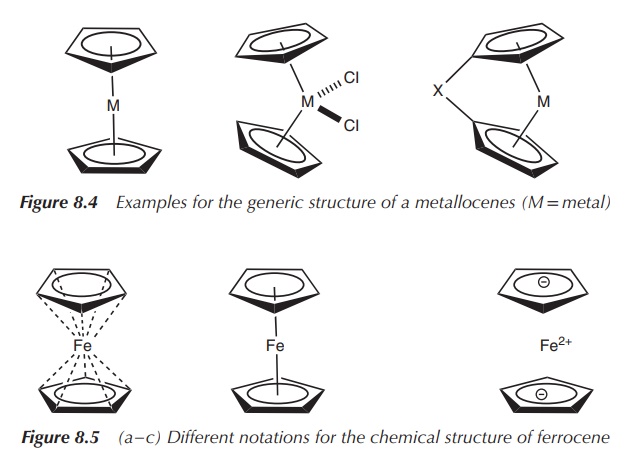
Sandwich complexes are defined as organometallic compounds
containing a central metal atom, which is bound by haptic
covalent bonds to two arene ligands.
The metal is typically placed between the two ring systems, which gives the complex the term sandwich.
Typically, sandwich structures are classified as parallel or
bent. Within a parallel sandwich structure, the two arene ligands are arranged
parallel to each other. In a bent sandwich structure, the two ligands are at an
angle to each other.
One of the best known metallocenes is ferrocene (η5-Cp)2Fe,
which is an iron (Fe2+)-based parallel sand-wich complex. The metal
centre is sandwiched between two cyclopentadienyl ligands, which are
coparallel. There are different ways of representing the structure of
ferrocene, as shown in Figure 8.5. The structure on (a) gives the best
understanding of the bonds present in ferrocene, whilst the structure on (b) is
the most commonly used one. The structure on (c) shows the electronic
properties of the individual components in ferrocene.
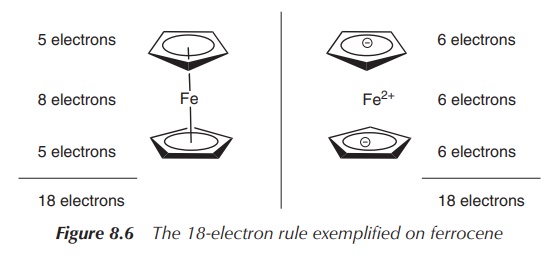
Ferrocene follows the so-called 18-electron rule, which most low-oxidation-state d-block metal organometallic complexes seem to follow. The number 18 is the effective number of electrons that a transition metal can accommodate in its nine valence orbitals (comprising five d orbitals, three p orbitals and one s orbital).
The 18-electron
rule is mainly used to predict
the formula for stable transition-metal complexes. The rule is based on the
principle that each transition metal has usually nine valence orbitals, one s-type, three p-type and five d-type orbitals. These interact with the ligands and accommodate up to 18 electrons. Transition-metal complexes containing 18
valence electrons (VEs) have the same electronic configuration as the noble gas
in this period, similar to the octet
rule described for main group metals.
Note that there are many examples known that do not follow the 18-electron rule.
Ferrocene is a good example to explain this
rule, and there are two ways of counting the electrons in ferrocene. On one
hand, it can be assumed that all partners are neutral, which means that
ferrocene consists of an Fe(0) centre and two Cp ligands. That means the Fe(0)
centre contributes eight electrons (group 8, eight VEs), whilst each Cp ligand
contributes five electrons. This adds up to a total of 18 electrons. On the
other hand, the second method of counting is based on the fact that each
partner carries a charge, which means an Fe2+ centre and two Cp−
ligands. Therefore the iron centre contributes six electrons and each Cp−
ligand also contributes six electrons, which sums up to also 18 electrons
(Figure 8.6).
It is important to note that this rule can be used to predict stable metal complexes, but it is only a formality. There are many examples of stable transition-metal complexes that do not follow the 18-electron rule, such as Pd(0) or Pt(0) complexes, or indeed some of the titanocenes and vanadocenes presented in the following.
Related Topics
