Vanadocenes
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Organometallic Chemistry
Vanadium, with chemical symbol V and atomic number 23, is a member of the d-block metals and belongs to group 5 of the periodic table of elements.
Vanadocenes
Vanadium, with chemical symbol V and atomic number 23, is a
member of the d-block metals and belongs to group 5 of the periodic table of
elements (Figure 8.28).
Vanadium can be found in the earth’s crust in numerous minerals and is isolated from ores mostly as a by-product. Its main application is in the steel industry, where it is used as an alloy in combination with iron. Vanadium pentaoxide is also being used as a catalyst for the production of sulfuric acid. The metal vanadium has very similar properties to titanium. Therefore, it is not surprising that its metallocene, vanadium dichloride, was also subjected to research as a potential anticancer agent.
Vanadium is easily passivated by an oxide film, and the metal is
insoluble in nonoxidising acids. Typical oxidation states are +II, +III, +IV
and +V, whilst the biologically active oxidation states are +IV and +V.
Vanadium reacts to vanadium halide by reacting the metal with the corresponding
halogen under heating, whilst it also reacts with oxygen with the formation of
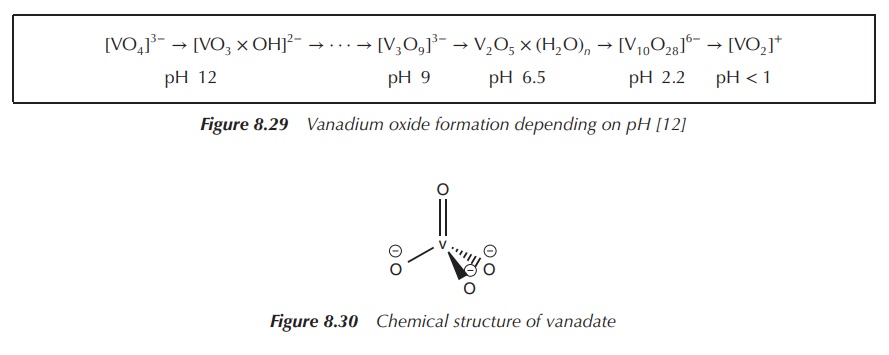
V2O5. Vanadium (+V) oxides are amphoteric and, as a result,
vanadates (VO43−) and dioxovanadium ions (VO2+)
are formed in aqueous solutions depending on the pH (Figure 8.29) .
Vanadium is an essential trace metal in the human body, but
still very little is known about its biological function. Vanadium is mainly
found in its ionic state bound to proteins. As mentioned, the metal mostly
occupies oxidation states +V and +IV in biological systems, resulting in
electron configurations of [Ar]3d0 for V+5 and [Ar]3d1
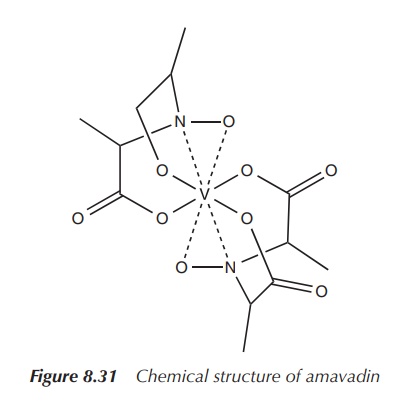
for V+4. The chemical formula for the tetrahedral ion vanadate is
written as VO43+; whereas the diatomic oxovanadium(+IV)
ion, also called vanadyl, has the
chemical formula VO2+ (Figure 8.30).
Vanadium compounds are well known for their
toxicity. The most famous example is the poisonous mush-room toadstool, Amanita muscaria. A. muscaria contains the toxic compound amavadin, which is a toxic
octahedral vanadium complex (see Figure 8.31) .
Vanadate and vanadyl are known to cause adverse effects in
mammals, including loss of body weight, gas-trointestinal problems,
reproductive toxicity and morbidity. However, their toxicity depends on a
variety of factors such as the chemical form, oxidation state, route of
administration and duration of exposure. Never-theless, toxic effects of
vanadate or vanadyl are observed only at dose levels significantly greater than
usual uptake through diet . Nevertheless, it is important to improve the
understanding of the adverse and toxic effects of vanadium compounds before any
compound can be successfully developed for clinical use.
Vanadocene dichloride as anticancer agents
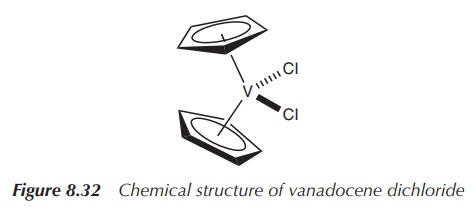
Vanadocene dichloride [(η5-C5H5)2VCl2,
dichloro bis( η5-cyclopentadienyl)vanadium(IV)] is structurally very
similar to Cp2TiCl2. It also consists of a metal centre
with an oxidation number of +IV, in this case vanadium, and two Cp−
and two chloride ligands. Vanadocene dichloride is a 17-electron complex
containing an unpaired electron and is therefore paramagnetic (Figure 8.32).
Vanadocene dichloride has found application as a catalyst for
polymerisation reactions, but was also intensively studied as an anticancer
agent in parallel to Cp2TiCl2 because of their structural
similarities. Vanadocene dichloride has proven to be even more effective than
its titanium analogue as an antiproliferative agent against both animal and
human cell lines in preclinical testing. The main problems are the difficult
characterisation of the active vanadium compounds and their fast hydrolysis.
Because of their paramagnetic character, it is difficult to apply standard
classical analysis techniques such as NMR (nuclear magnetic resonance) to
identify the antiproliferative vanadium species. Furthermore, vanadocene
dichloride undergoes fast hydrolytic processes and is even more prone to
hydrolysis than titanocene dichloride. This poses even more challenges for its
potential clinical application .
In recent years, researchers have shown renewed interest in the
use of substituted vanadocene dichlorides as potential anticancer agents. A
selection of substituted vanadocenes have been synthesised and tested for their
cytotoxic activity against testicular cancer. Examples of these compounds
include vanadocenes con-taining substituted cyclopentadienyl ligands and/or
replacement groups for the chloride ligands – similar to the research being
undertaken for cisplatin analogues. Results of in vitro studies show that these com-pounds exhibit good but
variable cytotoxic activity depending on the substitution pattern and induce
apoptosis (cell-induced cell death). Interestingly, only organometallic
vanadium(+IV) complexes showed cytotoxic activity against testicular cancer.
When the purely inorganic compound vanadyl(IV) sulfate was tested in the same
study, no cytotoxic effect was observed at the same concentrations. It is also
important to note that titanocene dichloride and other metallocenes had no
cytotoxic effect against testicular cancer. It was con-cluded that the mode of
action of vanadium-induced cytotoxicity must be different from that of
titanocene dichloride and other metallocenes (Figure 8.33) .
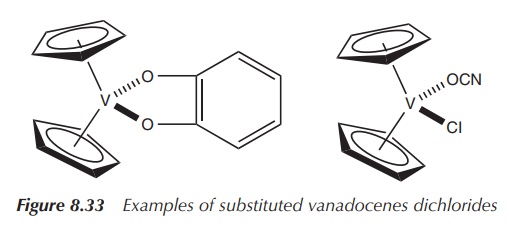
In parallel to the research undertaken with substituted titanocene dichlorides as potential chemotherapeutic agents, some of their vanadocene analogues have been synthesised. Some examples include the hydrolithiation of fulvenes and subsequent transmetallation with vanadium tetrachloride.
The resulting substituted vanadocene dichlorides were found to be highly toxic
compounds when tested in vitro
against a model of renal cell cancer and more potent than the corresponding
titanocene. Further preclinical studies are still needed (Figure 8.34) .
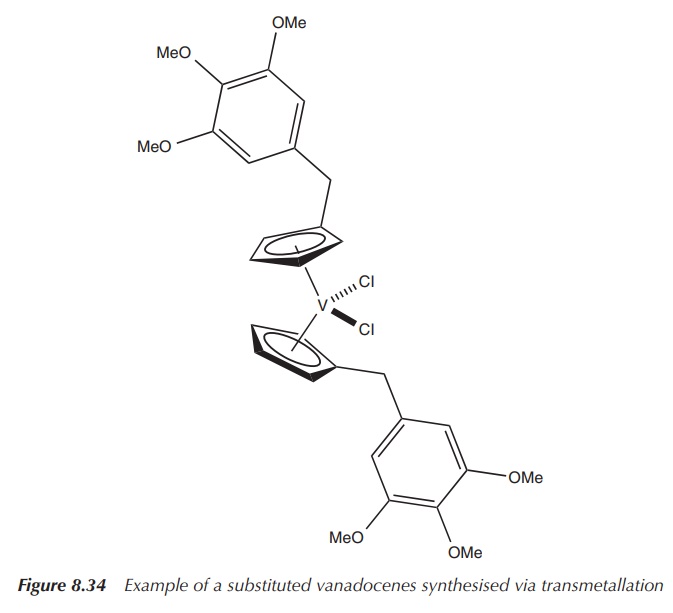
Further vanadium-based drugs: insulin mimetics
Towards the end of the nineteenth century, inorganic vanadium
compounds were under evaluation as potential treatment options for Diabetes
Mellitus (DM) as so-called insulin mimetics. Sodium vanadate (Na3V(+V)O4)
was tested for its ability to lower glucose levels in the blood of candidates
with and without DM. The inor-ganic vanadium compound showed mild effects in
some of the patients suffering from DM, whilst no severe
However,
research focused more on the less toxic inorganic vanadium compounds, such as
vanadyl sulfate (V(+IV)OSO4) which is significantly less toxic than
sodium vanadate. Nevertheless, with the development of insulin in 1922, the
interest in vanadium compounds as antidiabetic drugs diminished (Figure 8.35) .
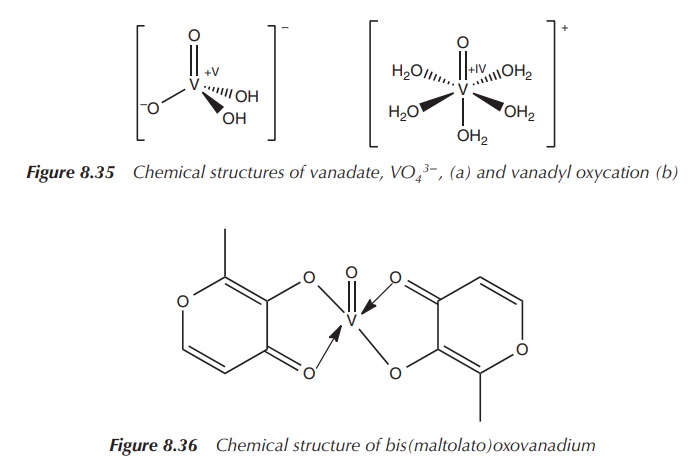
In more recent years, metal complexes have become of interest
for a variety of clinical applications. This also renewed the interest for
vanadium complexes to be examined for the treatment of diabetes. The vanadium
complexes bis(maltolato)oxovanadium (BMOV) and bis(ethylmaltolato)oxovanadium
(BEOV) have shown to be unique insulin mimetics when tested in diabetic rats
[9, 17]. An increase in uptake and tolerability compared to the inorganic form
was noted. Studies have also shown that there is a difference in distribution
between the inorganic and the complexed form of vanadyl in in vivo experiments, which might relate to the differences in
uptake and tolerability. Animal experiments with vanadyl sulfate have shown
accumulation of vanadium mainly in the kidneys and liver, whilst experiments
with the vanadyl complexes BMOV and BEOV resulted in a high accumulation on the
bones followed by kidneys (Figure 8.36) [14, 18].
BMOV has proven itself as a successful antidiabetic agent when
tested in animal models. Nevertheless, only very little is known about its mode
of action. It is believed that BMOV acts as a competitive and reversible
inhibitor of the enzyme protein tyrosine phosphatase (PTP). Other vanadium
complexes are also known to inhibit PTP, but mostly inhibiting it irreversibly .
PTPs belong a family of enzymes that remove phosphate groups
from phosphorylated tyrosine residues on proteins. Its member protein tyrosine
phosphatase 1B (PTP1B), which is located in the cytosol, has been iden-tified
as a negative regulator of insulin signal transduction. Resistance to insulin
can be observed in different tissues such as muscles, liver and fat, which are
all crucial for the homeostasis of glucose levels in the human body. In the
healthy human body, the transport of glucose into the cell occurs through the
activation of the insulin receptor including the phosphorylation of the
tyrosine residue. As a result, the so-called insulin recep-tor substrate (IRS)
is recruited, followed by the activation of several enzymes. Finally, the
glucose transporter GLUT4 is translocated, which mediates the transport of
glucose into the cell.
PTP1B seems to be a key regulator for the activity of the
insulin receptor, including all downstream sig-nalling processes . It works by
the dephosphorylation of the phosphotyrosine residues at the activated insulin
receptor kinase and therefore ultimately hinders the uptake of glucose. PTP1B
has been identified as a promising target for new drugs treating DM Type 2.
Blocking the PTP1B-mediated dephosphorylation of insulin receptor kinase by an
inhibitor of PTB1B is believed to lead to an increase in insulin sensitivity.
As previously mentioned, BMOV is believed to be a potent and
competitive inhibitor of the PTP1B activity and additionally seem to support
the autophosphorylation of the insulin receptor leading to an increased
sensi-tivity towards insulin. Research has shown that varying the organic
ligand has an influence on the effectiveness and bioavailability of the
resulting vanadium compound. It is believed that factors such as absorption,
tissue uptake and distribution are affected most. Interestingly enough, X-ray
crystal data of PTP1B soaked with BMOV showed only vanadate [V(+V)O43−]
at the active site. This would emphasise that the organic lig-ands are only
carriers of the active compound and play no role in the enzyme inhibition
itself. Furthermore, in aqueous solution, V(IV) is rapidly and reversibly
oxidised to V(V), supporting the possible formation of vanadate.
BEOV entered clinical trials and successfully finished phase IIa trials for the treatment of DM Type 2. In phase I trials, doses of 10–90 mg were given to healthy nondiabetic volunteers and no adverse side effects were seen. In the phase IIa clinical trial, seven diabetic patients were treated with 20 mg/day of BEOV and showed a reduction of around 15% of their blood glucose levels. Two patients were treated with a placebo, and no reduction in blood glucose levels was observed. It was also interesting to note that the glucose level reduction lasted for 1 week after finishing the treatment .
Related Topics
