Types of emulsions
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Dosage forms - Emulsions
Emulsions typically consist of a polar (e.g., aqueous) and a relatively nonpolar (e.g., an oil) liquid phase. Based on the nature of the internal and/external phase, emulsions can be classified into different types.
Types of emulsions
Emulsions
typically consist of a polar (e.g., aqueous) and a relatively nonpo-lar (e.g.,
an oil) liquid phase. Based on the nature of the internal and/external phase,
emulsions can be classified into different types (Figure
17.1).
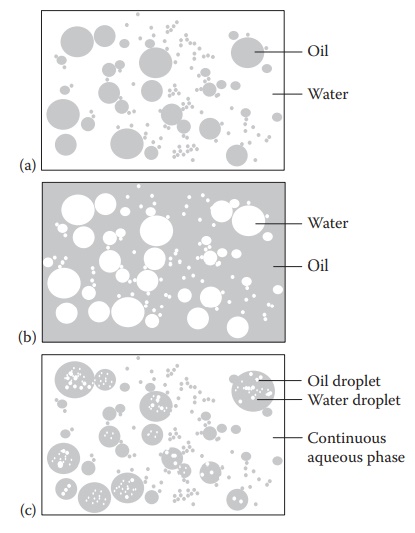
Figure 17.1 Types of emulsions: (a) o/w emulsions, (b) w/o emulsion, and (c) w/o/w multiple emulsion.
1. Oil-in-water emulsion
When
the oil phase is dispersed as globules throughout an aqueous con-tinuous phase,
the system is referred to as an oil-in-water (o/w) emulsion. An o/w emulsion is
generally formed if the aqueous phase constitutes more than 45% of the total
weight and a hydrophilic emulsifier, such as sodium lauryl sulfate,
triethanolamine stearate, sodium oleate, and glyceryl monostearate is used. The
emulsifier is present in the external,
2. Water-in-oil emulsion
When
the aqueous phase is dispersed, and the oil phase is the continuous phase, the
emulsion is termed as water-in-oil (w/o) emulsion. A lipophilic emulsifier is
used for preparing w/o emulsions. The w/o emulsions are used mainly for
external applications and may contain one or several of the following
emulsifiers: calcium palmitate, sorbitan esters (Spans), cholesterol, and wool
fats. Thus, the use of a lipophilic emulsifier enables the formation of w/o
emulsions with the oil phase as the external, con-tinuous phase.
3. Multiple emulsions
Multiple
emulsions are emulsions whose dispersed phase contains droplets of another
emulsion. Both water-in-oil-in-water (w/o/w) and oil-in-water-in-oil (o/w/o)
multiple emulsions are of interest as delayed- and/or sustained-action drug
delivery systems. They also have applications in cosmetics. Emulsifying a w/o
emulsion using water-soluble surfactants (which stabilize an oily dis-persed
phase) can produce w/o/w emulsions with an external aqueous phase, which
generally has a lower viscosity than the primary w/o emulsion. Multiple
emulsions can also be used for the encapsulation of peptides/proteins and
hydrophilic drugs.
4. Microemulsions
Microemulsions
are visually homogeneous, transparent/isotropic systems of low viscosity. In
their simplest form, microemulsions are small droplets (diameter 5–140 nm) of
one liquid dispersed throughout another by virtue of the presence of a fairly
large amount of surfactant(s) and cosolvent(s). Microemulsions have a very
finely subdivided dispersed phase, and often contain a high concentration of
the emulsifier(s) and a cosolvent (such as ethanol).
Microemulsions
are thermodynamically stable for prolonged periods of time. They can be
dispersions of o/w or w/o. The type of microemulsion (w/o or o/w) formed is
determined largely by the nature of the surfactants. Microemulsions can be used
to increase the bioavailability of poorly water-soluble drugs by incorporating
them into the oily phase. Incorporation of etoposide and methotrexate diester
derivative into w/o microemulsion has been suggested as a potential carrier for
cancer therapy.
Self-emulsifying drug delivery systems and self-microemulsifying drug delivery systems
A
solution of drug in the oil–surfactant–cosolvent mixture can sponta-neously
form an emulsion or microemulsion with minimal agitation at room temperature.
Whether this mixture forms an emulsion or a micro-emulsion depends on the
composition of this mixture and the amount of water added. A higher proportion
of oil and a lower proportion of cosolvent lead to the formation of an
emulsion. Self-microemulsifying mixtures typically contain a higher proportion
of the cosolvent and the surfactant, whereas the proportion of oil is lower.
These mixtures are termed as self-emulsifying drug delivery system (SEDDS) or
self-microemulsifying drug delivery systems (SMEDDS). The SEDDS and SMEDDS can
be administered orally for in vivo
emulsion or micro-emulsion formation in the patient’s GI tract. For example,
cyclospo-rine is available as a self-microemulsifying preconcentrate (Neoral®),
which is more rapidly and consistently absorbed than the original
self-emulsifying formulation of cyclosporine (Sandimmune®). Both these show
greater and more consistent bioavailability than unformulated cyclosporine.
