Type 1 Diabetes
| Home | | Biochemistry |Chapter: Biochemistry : Diabetes Mellitus
Persons with T1D constitute less than 10% of the nearly 20 million known diabetics in the United States.
TYPE 1 DIABETES
Persons with T1D
constitute less than 10% of the nearly 20 million known diabetics in the United
States. The disease is characterized by an absolute deficiency of insulin
caused by an autoimmune attack on the β cells of the pancreas. In T1D, the
islets of Langerhans become infiltrated with activated T lymphocytes, leading
to a condition called insulitis. Over a period of years, this autoimmune attack
on the β cells leads to gradual depletion of the β-cell population (Figure
25.2). However, symptoms appear abruptly when 80%– 90% of the β cells have been
destroyed. At this point, the pancreas fails to respond adequately to ingestion
of glucose, and insulin therapy is required to restore metabolic control and
prevent life-threatening ketoacidosis. β-Cell destruction requires both a
stimulus from the environment (such as a viral infection) and a genetic
determinant that allows the β cells to be recognized as “nonself.” [Note: Among
monozygotic (identical) twins, if one sibling develops T1D, the other twin has
only a 30%–50% chance of developing the disease. This contrasts with T2D, in
which the genetic influence is stronger and, in virtually all monozygotic
twinships, the disease eventually develops in both individuals.]
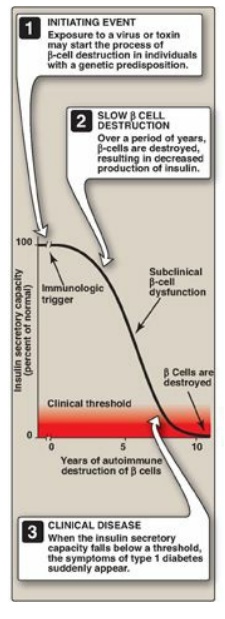
Figure 25.2 Insulin secretory
capacity during onset of type 1 diabetes. [Note: Rate of autoimmune destruction
of β cells may be faster or slower than shown.]
A. Diagnosis of type 1 diabetes
The onset of T1D is
typically during childhood or puberty, and symptoms develop suddenly. Patients
with T1D can usually be recognized by the abrupt appearance of polyuria
(frequent urination), polydipsia (excessive thirst), and polyphagia (excessive
hunger), often triggered by physiologic stress such as an illness. These
symptoms are usually accompanied by fatigue and weight loss. The diagnosis is
confirmed by a glycosylated hemoglobin concentration ≥ 6.5 mg/dl (normal is
less than 5.7), or a FBG ≥ 126 mg/dl (normal is 70–99). [Note: A FBG of 100–125
mg/dl is categorized as an impaired FBG. Individuals with impaired FBG are
considered “prediabetic” and are at increased risk for developing T2D.] Fasting
is defined as no caloric intake for at least 8 hours. Diagnosis can also be
made on the basis of a nonfasting (random) blood glucose level greater than 200
mg/dl in an individual with symptoms of hyperglycemia. [Note: The oral glucose
tolerance test, in which blood glucose is measured 2 hours after ingestion of a
solution containing 75 g of glucose, also is used but is less convenient. It is
most typically used to identify pregnant women with gestational diabetes.]
When blood glucose is greater than 180 mg/dl, the
ability of the kidneys to reclaim glucose is impaired. This results in glucose
“spilling” into the urine. The loss of glucose is accompanied by the loss of
water, resulting in the characteristic polyuria (with dehydration) and polydipsia
of diabetes.
B. Metabolic changes in type 1 diabetes
The metabolic
abnormalities of T1D mellitus result from a deficiency of insulin that
profoundly affects metabolism in three tissues: liver, muscle, and adipose (
Figure 25.3).
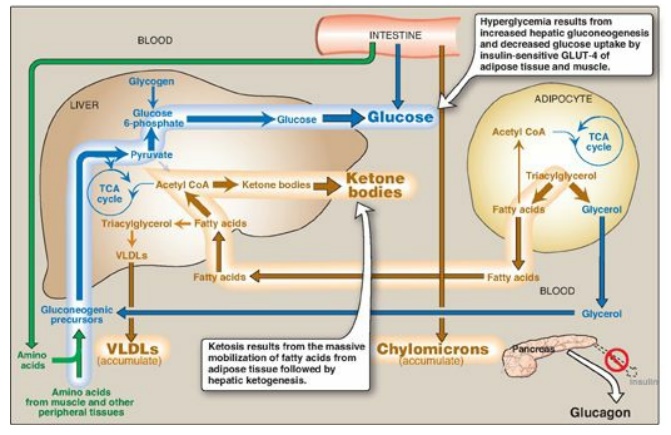
Figure 25.3 Intertissue
relationships in type 1 diabetes. TCA = tricarboxylic acid; CoA = coenzyme A;
VLDLs = very-low-density lipoproteins; GLUT = glucose transporter.
1. Hyperglycemia and ketoacidosis: Elevated levels of blood glucose
and ketone bodies are the hallmarks of untreated T1D (see Figure 25.3).
Hyperglycemia is caused by increased hepatic production of glucose via
gluconeogenesis, combined with diminished peripheral utilization (muscle and
adipose tissue have the insulin-sensitive glucose transporter GLUT-4;). Ketosis
results from increased mobilization of fatty acids (FAs) from adipose tissue,
combined with accelerated hepatic FA β-oxidation and synthesis of
3-hydroxybutyrate and acetoacetate. [Note: Acetyl coenzyme A from β-oxidation
is the substrate for ketogenesis and the allosteric activator of pyruvate
carboxylase, a gluconeogenic enzyme.] Diabetic ketoacidosis (DKA), a type of
metabolic acidosis, occurs in 25%–40% of those newly diagnosed with T1D and may
recur if the patient becomes ill (most commonly with an infection) or does not
comply with therapy. DKA is treated by replacing fluid and electrolytes and
administering short-acting insulin to gradually correct hyperglycemia without
precipitating hypoglycemia.
2. Hypertriacylglycerolemia: Not all of the FAs flooding the liver can be disposed of through oxidation or ketone body synthesis. These excess fatty acids are converted to triacylglycerol (TAG), which is packaged and secreted in very-low-density lipoproteins ([VLDLs]). Chylomicrons are synthesized from dietary lipids by the intestinal mucosal cells following a meal. Because lipoprotein degradation catalyzed by lipoprotein lipase in the capillary beds of adipose tissue is low in diabetics (synthesis of the enzyme is decreased when insulin levels are low), the plasma chylomicron and VLDL levels are elevated, resulting in hypertriacylglycerolemia (see Figure 25.3).
C. Treatment of type 1 diabetes
Individuals with T1D
must rely on exogenous insulin delivered subcutaneously either by periodic
injection or continuous pump-assisted infusion to control the hyperglycemia and
ketoacidosis. Two therapeutic regimens are currently in use, standard and
intensive insulin treatment.
1. Standard treatment versus intensive treatment: Standard treatment typically consists of one or two daily injections of recombinant human insulin. Mean blood glucose levels obtained are typically in the 225–275 mg/dl range, with a glycosylated hemoglobin (HbA1c) level of 8%–9% of the total hemoglobin (blue arrow in Figure 25.4). [Note: The rate of formation of HbA1c is proportional to the average blood glucose concentration over the previous 3 months. Thus, HbA1c provides a measure of how well treatment has normalized blood glucose in the diabetic patient over that time.] In contrast to standard therapy, intensive treatment seeks to more closely normalize blood glucose through more frequent monitoring and subsequent injections of insulin, typically three or more times a day. Mean blood glucose levels of 150 mg/dl can be achieved, with HbA1c approximately 7% of the total hemoglobin (see red arrow in Figure 25.4). [Note: Normal mean blood glucose is approximately 100 mg/dl, and HbA1c is 6% or less (see black arrow in Figure 25.4).] Therefore, normalization of glucose values (euglycemia) is not achieved even in intensively treated patients. Nonetheless, patients on intensive therapy show a 50% or more reduction in the long-term microvascular complications of diabetes (that is, retinopathy, nephropathy, and neuropathy) compared with patients receiving standard care. This confirms that the complications of diabetes are related to an elevation of plasma glucose.
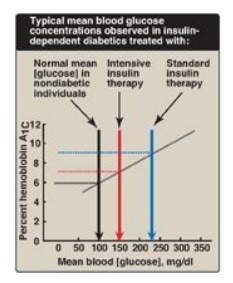
Figure 25.4 Correlation between mean blood glucose and hemoglobin A1C in patients with type 1 diabetes.
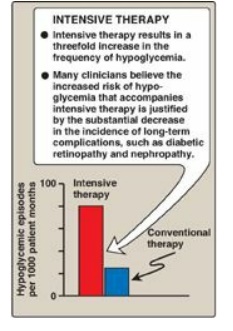
Figure 25.5 Effect of tight
glucose control on hypoglycemic episodes in a population of patients on
intensive therapy or conventional therapy.
2. Hypoglycemia in type 1 diabetes: One of the therapeutic goals in
cases of diabetes is to decrease blood glucose levels in an effort to minimize
the development of long-term complications of the disease. However, appropriate
dosage of insulin is difficult to achieve. Hypoglycemia caused by excess
insulin is the most common complication of insulin therapy, occurring in over
90% of patients. The frequency of hypoglycemic episodes, coma, and seizures is
particularly high with intensive treatment regimens designed to achieve tight
control of blood glucose (Figure 25.5). Recall that in normal individuals,
hypoglycemia triggers a compensatory secretion of counterregulatory hormones,
most notably glucagon and epinephrine, which promote hepatic production of
glucose. However, patients with T1D also develop a deficiency of glucagon
secretion. This defect occurs early in the disease and is almost universally
present 4 years after diagnosis. These patients, therefore, rely on epinephrine
secretion to prevent severe hypoglycemia. However, as the disease progresses,
T1D patients show diabetic autonomic neuropathy and impaired ability to secrete
epinephrine in response to hypoglycemia. The combined deficiency of glucagon
and epinephrine secretion creates a symptom-free condition sometimes called
“hypoglycemia unawareness.” Thus, patients with long-standing T1D are
particularly vulnerable to hypoglycemia. Hypoglycemia can also be caused by
strenuous exercise. Exercise promotes glucose uptake into muscle and decreases
the need for exogenous insulin. Patients are advised, therefore, to check blood
glucose levels before or after intensive exercise to prevent or abort
hypoglycemia.
3. Contraindications for tight control: Children are not put on a program of tight control of blood glucose before age 8 years because of the risk that episodes of hypoglycemia may adversely affect brain development. Elderly people typically do not go on tight control because hypoglycemia can cause strokes and heart attacks in this population. Also, the major goal of tight control is to prevent complications many years later. Tight control, then, is most worthwhile for otherwise healthy people who can expect to live at least 10 more years. [Note: For most nonpregnant adults with diabetes, the individual treatment strategies and goals are based on the duration of diabetes, age/life expectancy, and known comorbid conditions.]
Related Topics
