The Theory of Drying
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Drying
Theories of drying are limited in application in that drying times are normally experimentally determined.
THE THEORY OF DRYING
Theories
of drying are limited in application in that drying times are normally
experimentally determined. Nevertheless, an appreciation of the scope and
limitations of the different drying methods is given. The following terms are
employed in discussing drying: humidity and humidity of saturated air, relative
humidity, wet bulb temperature, and adiabatic cooling line. Other terms may be
defined as follows:
Moisture
content: This is usually expressed as a weight per unit weight of dry solids.
Equilibrium moisture content: If a material is exposed to air at a given
temperature and humidity, it will gain or lose moisture until equilibrium is
reached. The moisture present at this point is defined as the equilibrium
moisture content for the given exposure conditions. At a given temperature, it
will vary with the partial pressure of the water vapor in the surrounding
atmosphere. This is shown for a hypothetical hygroscopic material in Figure 7.1
in which the equilibrium moisture content is plotted against the relative humidity.
Any moisture present in excess of the equilibrium moisture content is called
“free water.”
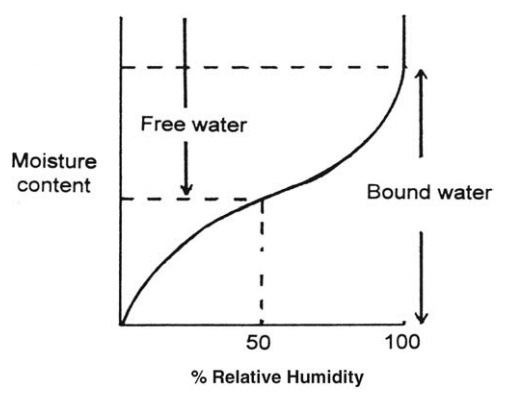
FIGURE 7.1 The relation between equilibrium
moisture content and relative humidity for a hygroscopic solid.
Equilibrium
moisture content curves vary greatly with the type of material examined.
Insoluble, nonporous materials, such as talc or zinc oxide, give equilibrium
moisture contents of almost zero over a wide humidity range. A moisture content
of between 10% and 15% may be expected for cotton fabrics under normal
atmospheric conditions. Drying below the equilibrium moisture content for room
conditions may be deliberately undertaken, particularly if the material is
unstable in the presence of moisture. Subsequent storage conditions then become
important for product stability.
The
equilibrium moisture content at 100% relative humidity represents the minimum
amount of water associated with the solid that still exerts a vapor pressure
equal to a separate water surface. If the humidity is reduced, only part of the
water evaporates before a new equilibrium is established. The water retained at
less than 100% relative humidity must, therefore, exert a vapor pressure below
that of a dissociated water surface. Such water is called “bound water.” Unlike
the equilibrium moisture content, bound water is a function of the solid only
and not of the surroundings. Such water is usually held in small pores bound
with highly curved menisci, is present as a solution, or is adsorbed on the
surface of the solid.
The
value of equilibrium moisture content curves is illustrated by the examples
given in Figure 7.2. The equilibrium moisture content of the antacid granules,
composed of magnesium trisilicate granulated with syrup, is a sen-sitive
function of relative humidity. If it is to be dried to a moisture content
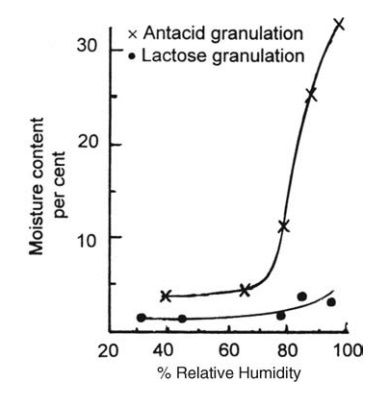
FIGURE 7.2 Equilibrium moisture content
curves for two tablet granulations.
With knowledge of the humidity
of the circulating air, psychrometric charts may be used to determine the
minimum air temperature that will dry the material to the required standard.
(In fact, the temperature has an effect on the equilibrium moisture content
that is independent of the humidity, but this can be neglected to a first
approximation.)
The lactose
granulation, on the other hand, has a low sensitivity to relative humidity.
Drying at low relative humidities derived from high air temperatures causes
only a marginal decrease in the final moisture content, and the stability of
the active ingredients associated with the lactose filler could be impaired.
This argument may only be applied to the final moisture content. It is not
related to the rate of drying that would, of course, be greater at higher
temperatures and lower humidities.
The effects of
storage after drying may also be assessed from the equi-librium moisture
content curves. Storage conditions are not critical for the lac-tose
granulation. If the antacid formulation was stored at a relative humidity of
only 65%, it would, given sufficient time, absorb moisture until the content
was 9%. This could be associated with poor flow characteristics and its
attendant difficulties during compression.
Dynamic vapor
sorption techniques now exist, which allow thorough studies of moisture
association with solids under a wide range of relative humidity conditions
based on microbalance technology.
Related Topics
