The Measure of Pressure Intensity
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Fluid Flow
The density term should be the difference between the density of the liquid in the column and the density of the surrounding air.
THE MEASURE OF PRESSURE
INTENSITY
Application
of equation (2.3) to the column of liquid shown in Figure 2.3A gives
PA
- P1 = -ρgh
and
P1
= PA + ρgh (2:4)
The
density term should be the difference between the density of the liquid in the
column and the density of the surrounding air. The latter is relatively small,
and this discrepancy can be ignored. P1 is the absolute pressure at
the point indicated, and PA is the atmospheric pressure. It is often
convenient to refer to the pressure measured relative to atmospheric pressure,
that is, P1 - PA. This is called the gauge pressure and
is equal to ρgh. SI unit for pressure is N/m2. Alternatively, the
gauge pressure can be expressed as the height or head of a static liquid that
would produce pressure.
Figure
2.3A represents the simplest form of a manometer, a device widely used for the
measurement of pressure. It consists of a vertical tube tapped into the
container of the fluid being studied. In this form, it is confined to the
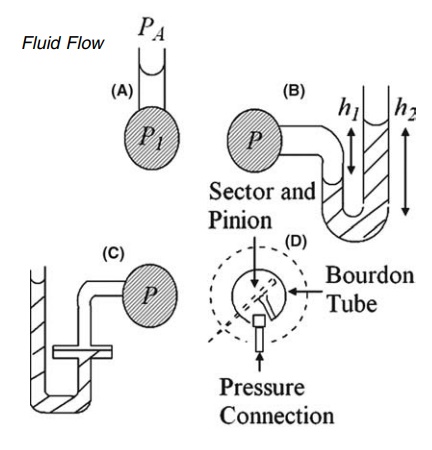
FIGURE 2.3 Pressure measurement apparatus
(A) vertical; (B) U-tube; (C) modified U-tube manometers and (D) Bourdon gauge.
pressure
measurement of liquids. This device is unsuitable for the measurement of very
large heads, because of unwieldy construction, or very small heads, because of
low accuracy. The U-tube manometer, shown in Figure 2.3B, may be used for the
measurement of higher pressures with both liquids and gases. The density of the
immiscible liquid in the U-tube, ρ1, is greater than the density of
the fluid in the container, ρ2. The gauge pressure is given by
P
= h1ρ1g
- h2ρ2g
The disadvantage of
reading two levels may be overcome by the modification in Figure 2.3C. The
cross-sectional area of one limb is many times larger than that of the other,
and the vertical movement of the heavier liquid in the wider arm can be
neglected and its level is assumed to be constant.
Sloping the reading
arm of the manometer can increase the accuracy of the pressure determination
for small heads with any of the manometers just described. The head is now
derived from the distance moved along the tube and the angle of the slope.
The Bourdon gauge, a
compact instrument widely used for the measure-ment of pressure, differs in
principle from the manometer. The fluid is admitted to a sealed tube of oval
cross section, the shape of which is shown in Figure 2.3D. The straightening of
the tube under internal pressure is opposed by its elasticity. The movement to
an equilibrium position actuates a recording mechanism. The gauge is calibrated
by an absolute method of pressure measurement.
The
principles of pressure measurement also apply to fluids in motion. However, the
presence of the meter should minimize perturbation in flow. A calming section,
in which a flow regime becomes stable, is present upstream from the pressure
tapping, and the edge of the latter should be flush with the inside of the
container to prevent flow disturbance.
FIGURE 2.4 Flow of a fluid past a cylinder.
Related Topics
