The Cytoplasmic Membrane - Mechanisms of action of antibiotics and synthetic anti-infective agents
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Mechanisms of action of antibiotics and synthetic anti-infective agents
The integrity of the cytoplasmic membrane is vital for the normal functioning of all cells. Bacterial membranes do not contain sterols and in this respect differ from membranes of fungi and mammalian cells.
THE CYTOPLASMIC
MEMBRANE
Composition And Susceptibility Of Membranes To Selective Disruption
The integrity
of the cytoplasmic membrane is vital for the
normal functioning of all cells.
Bacterial membranes do not contain
sterols and in this respect
differ from membranes of fungi and mammalian cells. Fungal membranes contain predominantly ergosterol as the sterol
component whereas mammalian cells contain cholesterol.
Gram-negative bacteria contain an additional outer-membrane structure that
provides a protective penetration barrier to potentially harmful
substances, including many antibiotics. The outer membrane
has an unusual asymmetric structure in which phospholipids occupy the inner face and the lipopolysaccharide (LPS) occupies the outer face. The outer membrane
is attached to the peptidoglycan by proteins and lipoproteins. The stability of all membranes is maintained by a combination of non-covalent interactions between the constituents involving ionic,
hydrophobic and hydrogen
bonding. The balance of these interactions can be disturbed
by the intrusion of molecules
(membrane-active agents) which destroy the integrity of the membrane, thereby causing
leakage of cytoplasmic contents or impairment of metabolic
functions associated with the membrane. Most membrane-active agents that function
in this way, e.g.
the alcohols, quaternary ammonium compounds and bisbiguanides have very
poor selectivity. They
cannot be used
systemically because of their
damaging effects upon mammalian cells; instead
they are used as skin antiseptics, disinfectants and preservatives. A few agents can be used therapeutically: the polymyxins (colistin), which act principally
upon the outer membrane
of Gram-negative bacteria,
and the antifungal
polyenes, which act upon fungal membranes. Other antifungal agents,
the imidazoles, triazoles and terbinafine act by blocking the synthesis of ergosterol, the major sterol present
in fungal membranes.
Polymyxins
Polymyxin E (colistin) is used in the treatment of serious Gram-negative
bacterial infections, particularly those
caused by Pseudomonas
aeruginosa. It binds
tightly to the lipid A component
of LPS in the outer membrane of Gram-negative
bacteria. The outer leaflet of the membrane structure is distorted, segments
of which are released and the permeability barrier
is destroyed. The polymyxin molecules can then penetrate to the cytoplasmic membrane where they bind to phospholipids, disrupt membrane integrity, and cause irreversible leakage of cytoplasmic components. Their
detergent-like properties are a key feature of this membrane-damaging action,
which is similar to that
of quaternary ammonium
compounds. With increasing resistance to the major groups of antibiotics, some multi-resistant organisms (e.g. Acinetobacter species) remain sensitive only to membraneactive agents such as colistin. However, some Gram-negative bacteria produce LPS that does not
bind polymyxins (e.g. Bacteroides
species and Burkholderia
cenocepacia) while resistance can occur in some normally sensitive organisms such as E. coli and
Pseudomonas aeruginosa through modification of their LPS structure (e.g. by addition of aminoarabinose or
aminoethanol substituents to the lipid
A regions of their LPS).
Daptomycin
This negatively charged
bactericidal cyclic lipopetide binds to the surface of the Gram-positive bacterial cell
membrane. The binding
is dependent on calcium ions. The
acyl tail portion
of the compound inserts itself into the cytoplasmic membrane and drug molecules
aggregate together forming channels.
The leakage of potassium ions from the
cells results in inhibition of macromolecular synthesis and cell death.
Polyenes
Amphotericin B and
nystatin are the most commonly used members of this group of antifungal agents. They derive their action
from their strong
affinity towards sterols, particularly ergosterol. The hydrophobic polyene region binds to the hydrophobic sterol
ring system within fungal membranes. In so doing, the hydroxylated portion of the polyene is pulled into the membrane interior, destabilizing
the structure and causing leakage
of cytoplasmic constituents. It is possible
that polyene molecules associate together in the membrane to form
aqueous channels. The
pattern of leakage
is progressive, with
small metal ions
such as K+ leaking first, followed by larger amino acids and nucleotides. The internal pH of the cells
falls as K+ ions are released, macromolecules are degraded and the cells
are killed. The selective antifungal activity of the polyenes is poor,
depending on the higher affinity for ergosterol than cholesterol. Kidney
damage is a major
problem when polyenes are used systemically to treat severe fungal infections. The problem can be reduced,
but not eliminated by administration of amphotericin as a
lipid complex or liposome.
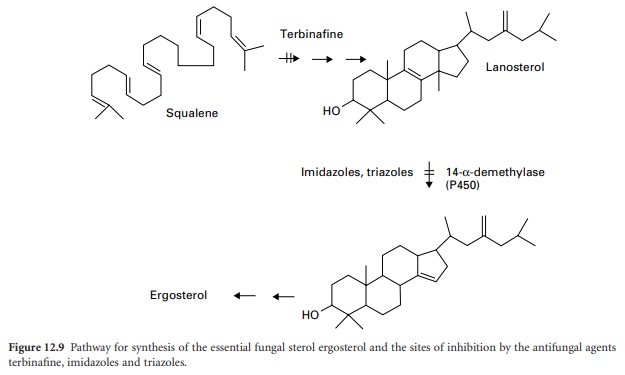
Imidazoles And Triazoles
The azole antifungal drugs act by inhibiting the synthesis
of the sterol components of the fungal
membrane (see also Chapter 4). They are inhibitors of one step
in the complex pathway of ergosterol synthesis
involving the removal of a methyl
group from lanosterol (Figure 12.9).
The 14-α-demethylase
enzyme responsible is dependent on cytochrome P-450. The imidazoles and triazoles cause rapid defects in fungal membrane integrity due to reduced levels of ergosterol, with loss of cytoplasmic constituents leading to similar effects
to the polyenes. The azoles are not entirely specific for fungal
ergosterol synthesis and have some action
on mammalian sterol
metabolism; for example, they
reduce testosterone synthesis.
Terbinafine
This synthetic
antifungal agent inhibits
the enzyme squalene epoxidase at an early
stage in fungal
sterol biosynthesis. Acting
as a structural analogue of squalene,
terbinafine causes the
accumulation of this unsaturated hydrocarbon,
and a decrease in ergosterol in the fungal cell membrane (Figure 12.9).
Related Topics
