Synthetic Strategy
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
The object of the game in organic synthesis is to assemble a particular molecule, the target, which has the correct carbon skeleton and the proper array of functional groups distributed on that skeleton.
SYNTHETIC STRATEGY
The
object of the game in organic synthesis is to assemble a particular molecule,
the target, which has the correct carbon skeleton and the proper array of
functional groups distributed on that skeleton. There are two general
strategies for accomplishing this synthetic challenge. The first is to start
with a molecule hav-ing the desired carbon skeleton and then manipulate the
functionality on that skeleton to that of the desired compound. The previous
chapter as well as many texts deals with functional group preparations and thus
provides methods for the installation and/or manipulation of functional groups
on the skeleton.
The
second general synthetic strategy is to assemble the proper carbon skeleton and
then adjust the functionality in the resulting molecule to that of the target.
Obviously assembling the carbon skeleton from smaller building blocks is a more
versatile and convergent approach because the carbon skeleton of the target may
not be available (or at least not readily available). Moreover, once the
synthetic sequence needed to assemble the carbon skeleton has been developed,
it can potentially be adapted to produce a series of structurally related
molecules. This is particularly useful when such a series of compounds is
needed to test for particular physical, chemical, or biological properties.
To
assemble needed carbon skeletons from smaller units, it is absolutely crucial
to be able to form carbon–carbon bonds between them. Consequently carbon–carbon
bond-forming reactions are among the most important organic reactions. Since
any particular carbon–carbon bond is merely a covalent link between two
skeletal fragments, synthesis of a target such as R3C–CR’3
can be accomplished by forming a carbon–carbon bond between the two skeletal
frag-ments R3C and CR’3. Since the bond to be made
contains two electrons that are shared, there are three modes by which these
electrons can be distributed between the two fragments to be joined.
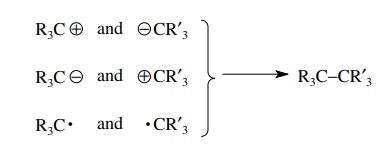
There
are two ionic modes of bond formation where one carbon fragment is
nucle-ophilic (e−
rich) and the other is electrophilic (e− poor). There is one free-radical
mode in which each fragment has a single unpaired electron which becomes shared
upon bond formation. To understand carbon–carbon bond-forming reac-tions that
take place by ionic or two-electron processes, we need to know what species or
compounds can serve as carbon-centered nucleophiles and what species or
compounds can serve as carbon-centered electrophiles. (Free-radical reactions
will be considered later.)
