Surface Tension
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Surface Tension
A surfactant is defined as a material that can reduce the surface tension of water at lower concentrations. This molecule is made up of water-soluble and water insoluble components.
Surface Tension INTRODUCTION A surfactant is defined as a material that can reduce the surface tension of water at lower concentrations. This molecule is made up of water-soluble and water insoluble components. Surface agent may enhance or retard the drug absorption, which depends upon the chemical nature of surfactant, its concentration, its effect on biological membrane, and micelle formation. At lower concentrations, the surfactant enhances the absorption rate; the same in higher concentrations reduce the absorption rate. In lower concentrations, they reduce the surface tension and bring about better absorption through better contact of the molecules with absorbing membrane, but when the concentration crosses the critical micelle concentration, the surfactant aligns them at the surface so that the hydrophilic end is towards the water and hydrophobic is squeezed away from the water. These molecular aggregates are called micelle, which entrap the drug molecule in their hydrophobic core, and result in the retardation of the rate of absorption (Fig. 6.1). The orientation of surface active molecules at the surface of water or at the interface of polar and nonpolar liquids takes place with the nonpolar (hydrocarbon) portion of the molecule oriented towards the nonpolar liquid or vapour phase and polar groups (e.g. -COOH, -OH, -NH2, -NO2, etc.) towards the polar liquid. Three forces are involved in the orientations of this type, namely, van der Waals forces, hydrogen bonds, and ion dipoles. A surfactant molecule exhibits two distinct regions of lipophilic and hydrophilic character, and such compounds are commonly categorized as amphiphilic or as amphiphils. Molecules of this type may vary markedly from predominantly hydrophilic to predominantly lipophilic, depending on the relative ratio of polar to nonpolar groups present.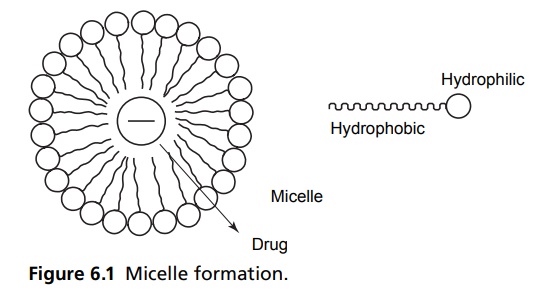
Related Topics
