Structural features of aminoglycoside antibiotics
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antibiotics
The aminoglycoside antibiotics contain two important structural features. They are amino sugar portion and centrally placed hexose ring, which is either 2-deoxystreptamine or streptidine.
The
aminoglycoside antibiotics contain two important structural features. They are amino
sugar portion and centrally placed hexose ring, which is either
2-deoxystreptamine or streptidine.
1. Amino sugar portion
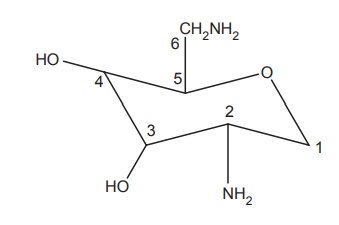
i. The bacterial inactivating enzymes targets C-6 and C-2 position,
and the substitution with methyl group at C-6 increases the enzyme resistance.
ii. Cleavage of
3-hydroxyl or the 4-hydroxyl or both groups does not affect the activity.
2. Centrally placed hexose ring (aminocyclitol ring)
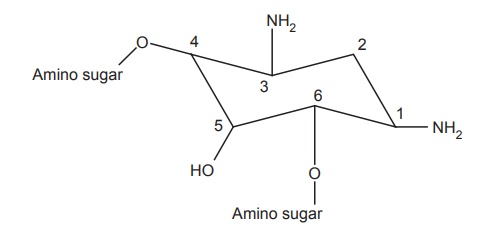
i.Various modifications at C-1 amino group have been tested. The
acylation (e.g. amikacyn) and ethylation (e.g. 1-N-ethylsisomycin) though does not increase the activity helps to
retain the antibacterial potency.
ii. In sisomicin
series, 2-hydroxylation and 5-deoxygenation result in the increased inhibition
of bacterial inactivating enzyme systems. Thus, very few modifications of the
central ring are possible, which do not violate the activity spectrum of
aminoglycosides.
Related Topics
