Sterilization Considerations
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Sterile Pharmaceutical Products
It is axiomatic that whatever method is chosen, the process should not cause damage to the product. By reference mostly to moist heat sterilization processes (the reader should remember that there are parallel approaches to other methods of sterilization) this section illustrates the factors that must be considered in the design of a sterilization process.
STERILIZATION CONSIDERATIONS
It is axiomatic that whatever method
is chosen, the process
should not cause
damage to the product. By reference mostly to moist
heat sterilization processes (the reader should
remember that there are
parallel approaches to other methods
of sterilization) this section illustrates the factors
that must be considered in the design of a sterilization process.
The simplest
method of sterilization, for an aqueous product, is to expose
it to the standard moist
heat sterilization conditions, i.e. holding the product at 121 °C for
15 minutes, a process termed
overkill. These conditions are quite severe and therefore
milder conditions might be
considered, i.e. a lower holding
temperature than 121 °C,
or a shorter holding
period than 15 minutes for a product prone to degradation. The minimum holding
period for moist heat sterilization might be considered to be 8 minutes at 121 °C. However, in reality a slightly shorter holding period may be satisfactory if the lethality of the whole
autoclave cycle (including heat-up and cooling phases) is calculated using F0 values and shown to afford the requisite SAL. F0 values of 8 minutes
or more
are normally considered satisfactory. Use of
lower temperatures and times gives an autoclave
process partly based on the initial bioburden and partly on the
known stability of the product.
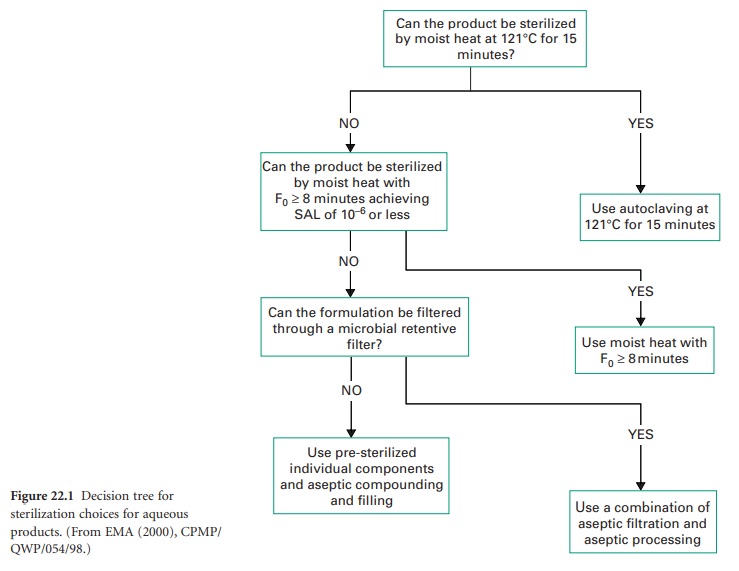
A) Decision Trees
Where it is not possible to sterilize a product in its final container by terminal heat
sterilization at 121
°C for 15 minutes, decisions have to be made
to use an alternative
method. The options include filtration in combination with aseptic
processing, but readers
should note that aseptic processing by itself is not a method
of sterilization, rather
of preventing contamination of the product whilst it is manufactured from
individually sterilized components.
The European Agency for the Evaluation of Medicinal
Products in 2000 produced an Annex for Guidance on Development Pharmaceuticals (CPMP/QWP/155/96) showing decision trees for the selection of sterilization
methods. The tree for the sterilization choices for aqueous products is shown
in Figure 22.1.
The initial premise
is that if the products
may be sterilized at 121 °C for 15
minutes, that process should
be used. The next alternative is that if the product
is stable when
an F0 of 8 minutes or more can be used,
then the reduced
moist heat process should be undertaken. If heat processes are unsuitable (an F0 < 8 minutes
will not achieve
the necessary SAL),
then filtration through
a microbial filter
should be chosen as the process to render the product sterile.
If that process cannot be utilized, then presterilizing of stable components and aseptic compounding and filling must
be considered. The described methods generally show
decreasing levels of sterility assurance on moving down
the tree. It is therefore imperative to remember that the highest
level of sterility assurance
is achieved in conjunction with the
lowest presterilization bioburden. The use of inappropriate heat-labile packaging material cannot by itself be the reason for the use of aseptic processing, and any manufacturer should use the best sterilization method achievable for a given
formulation before selecting the packaging
material. The manufacture of biotechnology products, which are typically
heat-labile peptides, proteins
or nucleic acids, will provide a
challenge as their overall stability dictates their
positioning near the bottom of the
decision tree. They may require
sterilization by submicron (< 0.1 μm) filtration and filling and finishing using aseptic processes. The overall
SAL for terminally sterilized products
should be less than 10−6 and for aseptically
produced products less than 10−3.
B) Problems Of Drug Stability
Certain issues
of product instability may be resolved by formulation or careful selection
of vehicle. Aminophylline injection, for example, is a solution of the drug
in Water for Injections Injections
free from
carbon dioxide, as the presence ![]()
![]() of this gas causes precipitation of the active
ingredient. Similarly, promethazine injection is a solution
of the active ingredient in Water for free
from dissolved air, as the presence of oxygen would cause promethazine
oxidation. Removal of these gases
can be accomplished by prior boiling;
additionally, the product may be packed under
an atmosphere of nitrogen to eliminate oxygen from
the headspace in the ampoule.
of this gas causes precipitation of the active
ingredient. Similarly, promethazine injection is a solution
of the active ingredient in Water for free
from dissolved air, as the presence of oxygen would cause promethazine
oxidation. Removal of these gases
can be accomplished by prior boiling;
additionally, the product may be packed under
an atmosphere of nitrogen to eliminate oxygen from
the headspace in the ampoule.
Formulations may be further
stabilized by the
inclusion of
inactive ingredients with specific functions.
Although the British Pharmacopoeia (2010) describes
chloramphenicol eye drops
as a sterile solution of chloramphenicol in purified water, normally
the system is buffered for stability with a boric
acid/sodium borate buffer (see Table 22.1). Sodium
metabisulphite may be found in many products as an
antioxidant to prevent degradation of the active, examples being
promethazine injection and
adrenaline injection. The presence of antimicrobial preservatives may be found
in multiple-dose products, to
prevent microbial growth following contamination during use. Many
of these formulation considerations relate to stability of the product
during storage, but an understanding of thermostability is required for the selection of the appropriate sterilization process.
The choice of sterilization method
depends on the thermostability of the active ingredient. Moist heat sterilization can only
be applied to drugs that
are heat-stable in aqueous
solution and are not subject
to hydrolysis. Where aqueous solutions are so unstable
that chemical stabilization
is impossible, consideration should be given to sterilization of the drug itself by dry heat processes
(160 °C for 2 hours or its equivalent at higher temperatures) in its final
container and dissolution immediately before use by the addition
of sterile Water for Injections
BP.
For drugs which
are both thermolabile and unstable in aqueous
solution, a sterile
solution of the drug may
be freeze-dried in its final container
and is again reconstituted as above just
before use. Examples
include many antibiotics
and Hyaluronidase Injection BP.
Related Topics
