Solved Problems on Mechanisms of Organic Reactions
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Mechanisms of Organic Reactions
Questions and Answers, Solved Problems on Mechanisms of Organic Reactions - Organic Chemistry : Mechanisms of Organic Reactions
PROBLEMS
5.1. The
base-promoted elimination of quaternary ammonium ions (Hofmann elimination) has
been proposed to proceed by an E2-like mechanism. Tell how each of the
following observations supports this mechanistic classifi-cation. Be specific
about exactly what each piece of information reveals.
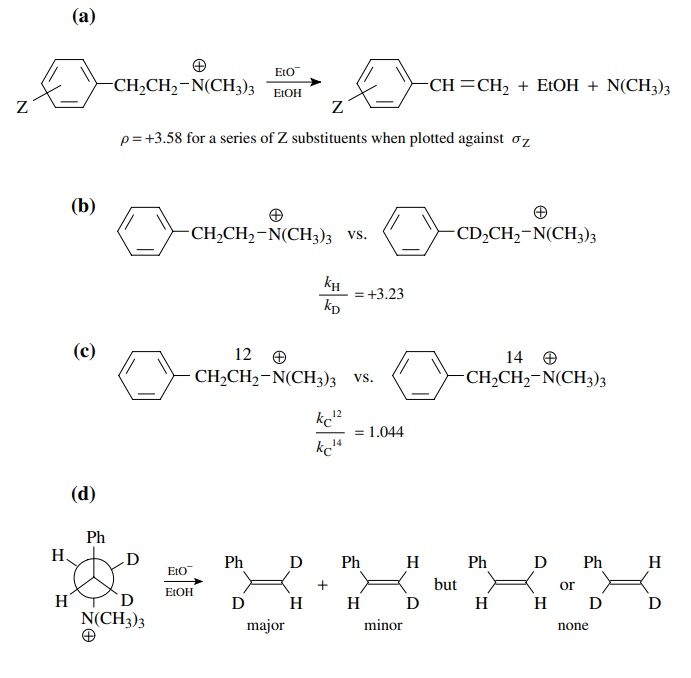
Answer:
(a)
The positive sign of ρ shows that an increase in electron density occurs during
the transition state of the rate-determining step. The magnitude shows that
there is a considerable electron density increase. Since the reagent
responsible is the base ethoxide, it is pretty clear that significant benzylic
proton removal by the base is occurring in the activated complex of the
rate-determining step. Moreover proton removal is well advanced.
(b)
This is a primary kinetic deuterium isotope effect, consistent with the notion
that removal of the benzylic proton by base is taking place in the
rate-determining step.
(c)
This is also a primary kinetic isotope effect; only this is a carbon isotope
effect. This means that cleavage of the carbon – nitrogen bond is occurring at
the transition state of the rate-determining step. Since both proton removal
and loss of the leaving group are occurring at the transition state, this then
seems to be a concerted E2-type elimination.
(d)
These results show that there is a distinct stereoelectronic requirement for
this process — that the proton and the leaving group are antiperiplanar. This
is typical of an E2-type base-promoted elimination. The major product is the
result of the deuterium isotope effect, which results in faster removal of the
proton than the deuterium.
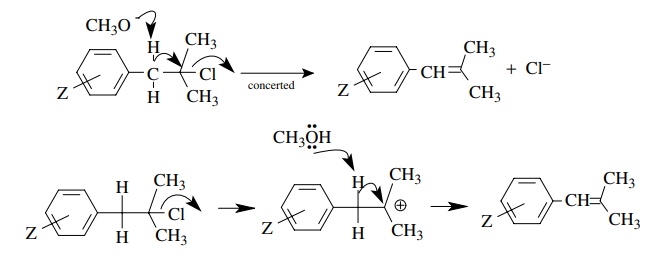
5.2. Explain the
mechanistic significance of the ρ
values for the elimination of 1 under the two different sets of conditions.
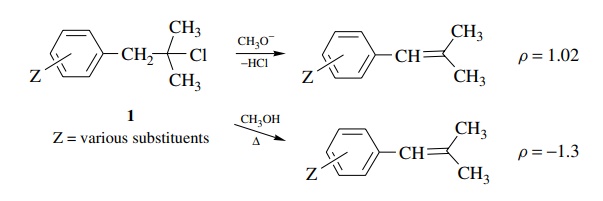
Answer:
The
positive value of ρ is indicative of increasing electron density on the
benzylic carbon at the transition state of the rate-determining step. This is
consistent with a mechanism in which the methoxide is acting as a base to
remove the benzylic proton in the rate-determining step. The small value
suggests that proton removal is not far advanced.
The second case with the negative value of ρ indicates that in this system a decrease in electron density occurs at the transition state. Ionization of the chloride leaving group to give a carbocation is consistent with this ρ value. The modest magnitude is consistent with the fact that charge formation occurs at the β position relative to the ring so the effect of substituents on charge formation is small. Also the correlation with σ is consistent since there is no direct resonance interaction of the developing charge with the aromatic ring due to the insulating methylene group.
5.3. Propose a
mechanism for the following reaction given that kH/ kD
= 6.1 and ρ− = 2.02 (there is a better correlation with σ − substituent constants than with σ values).
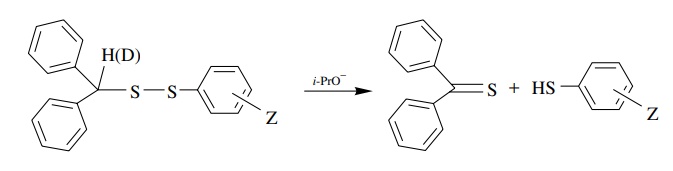
Answer:
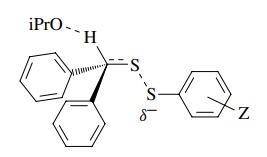
The positive sign, magnitude, and correlation with σ − indicate that there is a significant increase in electron density on an atom in conjugation with the aromatic ring. That would be the sulfur atom attached to the ring that is substituted. The primary kinetic deuterium isotope effect shows that cleavage of the C–H bond occurs in the rate-determining step. The best mechanism is a concerted, base-promoted elimination
5.4. Tell how you
could use isotopes to distinguish between the following two mechanisms:
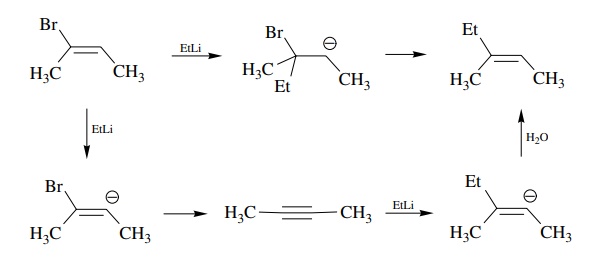
Answer:
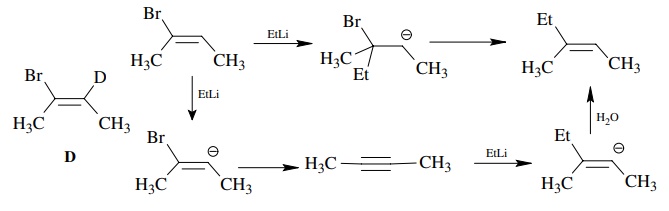
Since
the lower pathway has proton removal as an integral part of the mechanism, you
could use isotopic substitution in two ways. First make the deuterated
com-pound D. The kinetic deuterium
isotope effect should be primary for the lower path but very near 1 for the
upper path. Moreover, if you looked at the product, there should be no
deuterium left in the product if the lower path is followed, whereas deuterium
should be retained in the product if the addition elimination path is followed.
5.5. The following
transformations occur by different mechanisms. Show them and predict what a
Hammett study would show for each of them.
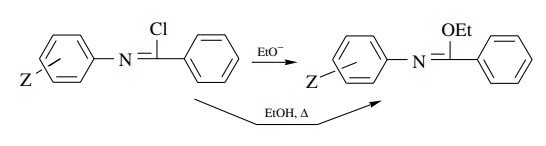
Answer:
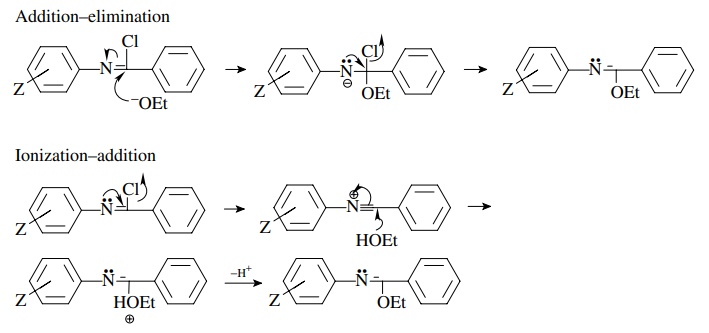
In
the first case the ρ value will be
positive because of the increase in electron density on the nitrogen next to
the aromatic ring and may correlate better with σ −
than with σ because of conjugation
with the ring. In the second case, ionization would lead to a decrease in
electron density on the nitrogen attached to the ring and would thus give a
negative r value.
5.6. The base
hydrolysis of a series of substituted phenoxy esters gave a much better
correlation when the rate constants were plotted against σZ− than against σZ.
Give the mechanistic significance of this behavior.
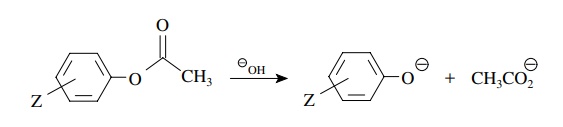
Answer:
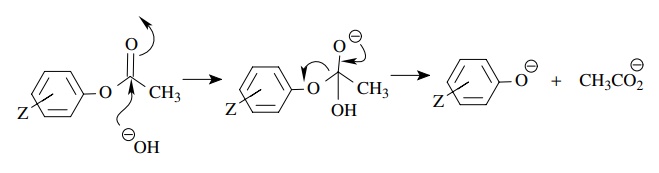
The
better correlation with σ − indicates that
increasing electron density in con-jugation with the aromatic ring is involved
in the rate-determining step. For the two mechanistic steps of the hydrolysis,
both should have positive ρ values
but only the second has charge development in conjugation with the ring. Thus
for this hydrolysis breakdown of the tetrahedral intermediate is rate determining.
5.7. Based on the
better correlation of rates versus σZ+
and ρ+ = −1.03, give a
likely mechanism for the following substitution:
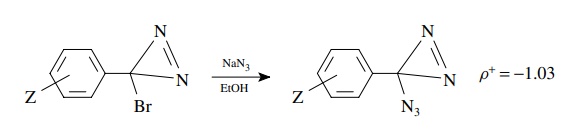
Answer:
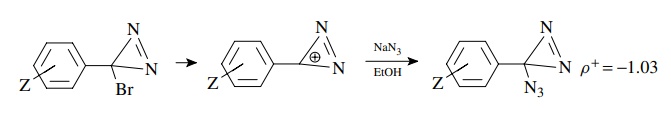
The
correlation with σ + and the negative
slope indicate charge deficiency formed in conjugation with the ring. This in
turn suggests an ionization mechanism in which ionization of bromide gives a
cation. This cation might be particularly favored because it is an aromatic
cation (Huckel) and would also explain the relatively low ρ+ value. The ρ + is low because the product cation
is stabilized; thus the transition state is early with little charge
development.
5.8. When 17α-methyl-5α-androstan-3β,17β-diol and its deuterated analog were
tested, it was found that the protio compound is three times more active than
the deutero analog. It is also known that the diol itself is not biologically
active. What is the likely metabolically active material? Explain.
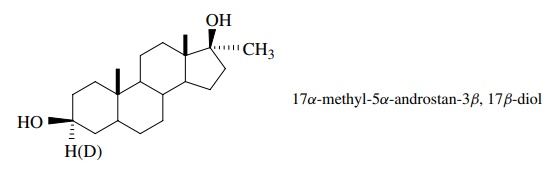
Answer:

The
difference in activity for the protio and deutero compounds is likely a kinetic
isotope effect of the transformation into the active compound. Since that
involves removal of the methine proton, a reasonable deduction is that the
ketone is the active species.
5.9. The oxidation
of substituted benzaldehydes to substituted benzoic acids by pyridinium
fluorochromate (PFC) has been studied, and it has been found that the reaction
is first order with respect to pyridinium fluorochromate but is of complicated
order with respect to the aldehyde. The following scenario was proposed to
account for this behavior:
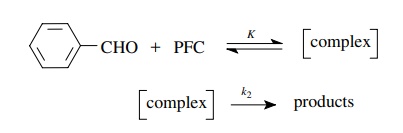
Using the
steady-state approximation, derive the rate law for this mecha-nistic scenario.
It was further found
using α-deutero benzaldehyde that kH/ kD = 5.33 and using substituted benzaldehydes
that ρ+ = −2.2:
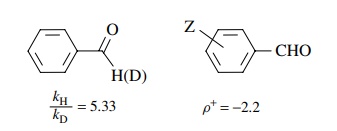
Use these data to
delineate the nature of the rate-determining step and propose a plausible
mechanism for the reaction. Explain how you used the data to arrive at the
mechanism.
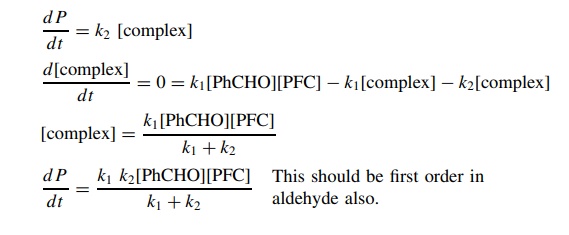
The
kinetic deuterium isotope effect indicates that C–H bond cleavage is part of
the rate-determining step and the negative ρ
value shows that there is electron defi-ciency being produced on the benzylic
carbon in the activated complex. Therefore
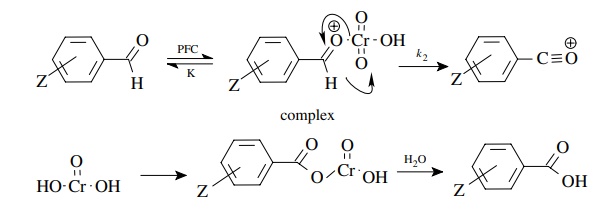
5.10. The rate of
reaction of substituted aromatic chlorides with methoxide to produce
substituted anisoles is found to give a linear correlation with σ − but not with σZ.
Explain in terms of a reaction mechanism.
Answer:
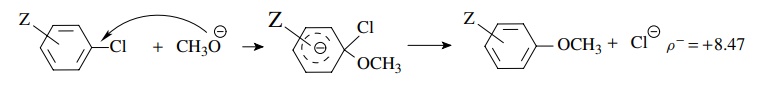
The correlation with σ − , the positive slope, and the relatively large magnitude of ρ means that a large charge density is built up on the ring in the activated complex of the rate-determining step. Addition of methoxide to give the Meisenheimer complex could account for this.
5.11. Derive the
rate law that would describe the rate of product formation for the following
reaction assuming that the cationic intermediate is a steady-state
intermediate:
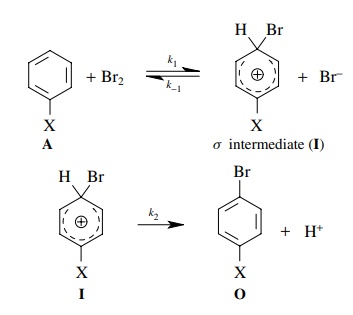
Answer:
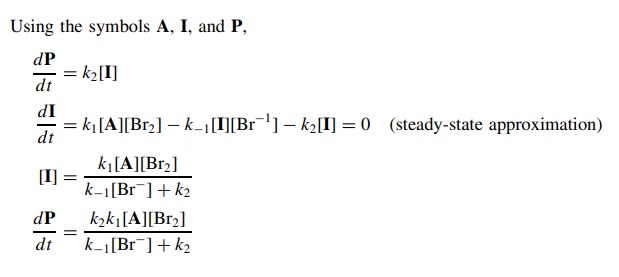
5.12. Derive the
rate law that would describe the rate of product formation for the following
reaction where no assumptions are made as to the relative magnitudes of k1, k−1, or k2:
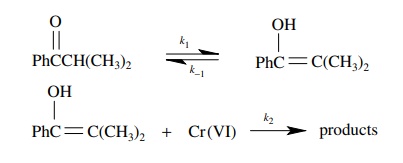
Answer:
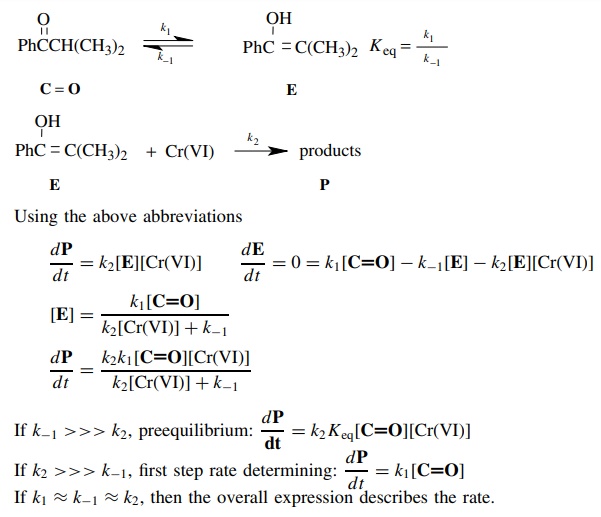
5.13. Benzaldehyde
cyanohydrin formation shown below may involve rate-determining attack by either
H+ or
CN−. A ρ value of +2.3 was found for
the rate of formation of cyanohydrins from a series of substituted
benzaldehydes. Which step is rate determining? Based on the fact that
cyanohydrin formation does not occur in basic solution, write a complete
mechanism for the process.
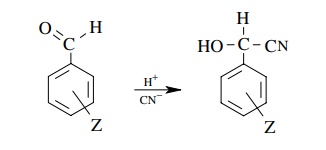
Answer:
Mechanism could be
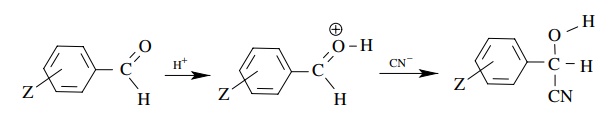
If
the protonation were rate determining, then a decrease in electron density at
the carbonyl carbon occurs. The ρ
value should be negative. If the second step is rate determining, then
nucleophilic addition should result in an increase in electron density at the
carbonyl group. the ρ value should be
positive. The data show that the second step is probably rate determining.
Moreover the lack of reaction under basic conditions means that the carbonyl
group must be activated by protonation for the cyanide to add. Thus the
protonation step is probably an equilibrium step.
5.14. For the
following reactions tell which should have kH/ kD values greater than 1.5
for isotopic substitution at the starred hydrogens:
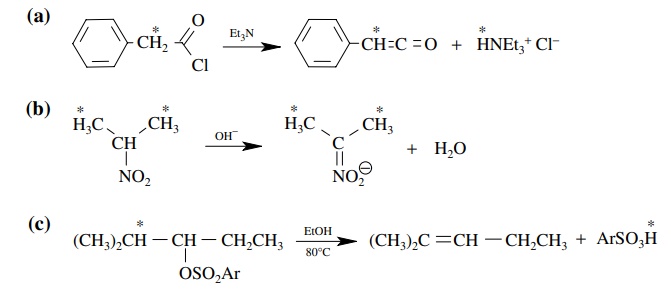
Answer:
(a)
Since a base is used in the reaction and the base apparently removes the α proton, there should be a primary
kinetic deuterium isotope effect of >1.5.
For this to be true, proton removal must occur in the rate-determining step.
(b) In this case the base removes a proton from the molecule
but the proton that is removed is not the one for which isotopic substitution
has been done. Thus there should be only secondary isotope effects for this
reaction of <1.5 (actually about
1.1).
(c)
In this reaction a proton is lost from the molecule in order to form the
product and that proton is one for which isotopic substitution is indicated.
However, since there is no good base present to cause its removal, it is likely
that the rate-determining step is ionization of the leaving group to give a
carbocation. The proton is lost from this cation in a subsequent step. Since
proton loss occurs after the rate-determining step, no primary kinetic
deuterium isotope effect will be seen. Thus the isotope effect will be <1.5. Actually there is a secondary
kinetic deuterium isotope effect of about 1.08.
5.15. For the
solvolyses of A and B, rates of ionization were found to correlate best with ρ+ and gave the ρ+
values shown.
(a) How do the
transition states for the solvolyses of A and B vary?
(b) How do you know
this and why?
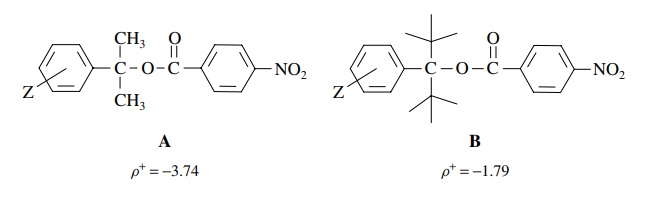
Answer:
(a) Since both correlate with σ + , there is a
development of positive charge on the benzylic
carbon which can be delocalized into the aromatic ring. The lower ρ value for B means that less electron deficiency is produced on the benzylic carbon of B than A.
(b)
Since both of these tertiary, benzylic substrates solvolyze by ionization as
the rate-determining step, the difference in the transition state parameters
suggests that the transition state for the tert-butyl
case B is much earlier. One reason
could be that the product ion from B
is more stable than the product ion from A,
which would result in an earlier transition state by the Hammond postulate. This is not a likely explanation since
both are tertiary benzylic carbocations as mentioned earlier. The difference in
ρ values is probably related to
steric factors. Since the bulky t
-butyl groups tend to flatten out the normal tetrahedral geometry, B is strained relative to A and thus of higher energy. (This
effect is actually termed B strain.) Thus the products are of similar energy
but the reactants are not. The Hammond postulate predicts that the reactant of
higher energy, B, will have an
earlier transition state, as is seen.
5.16. Draw
mechanisms for the following transformations using curved-arrow notation based
on the data you are given. There may be more than one step.
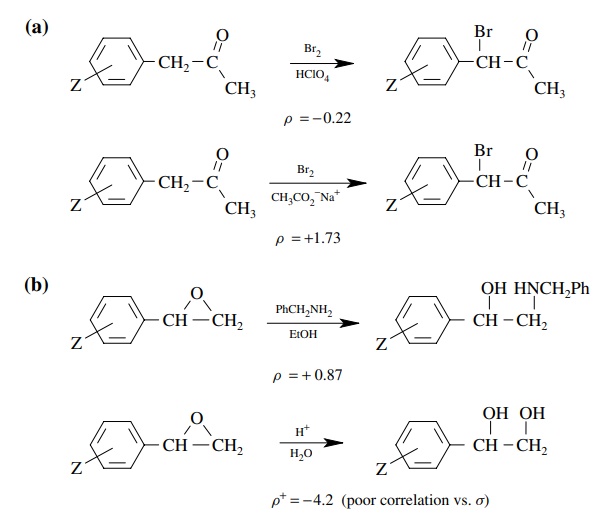
Answer:
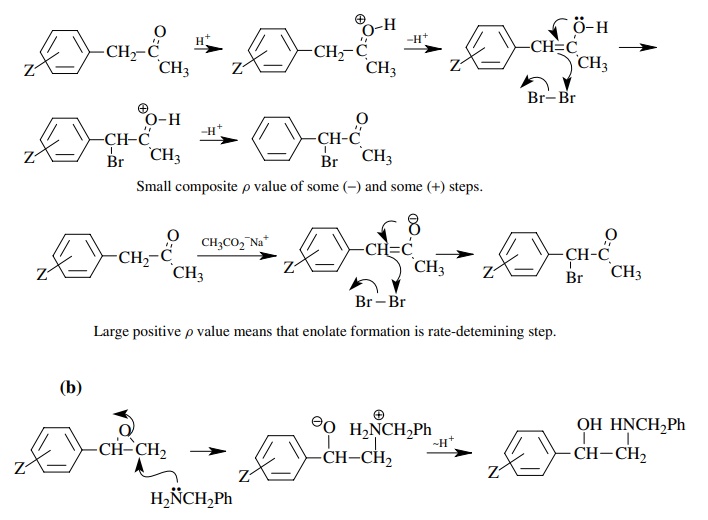
Positive
ρ value consistent with nucleophilic
addition to epoxide which increases electron density on oxygen. Small magnitude
consistent with negative charge being insulated from the ring.
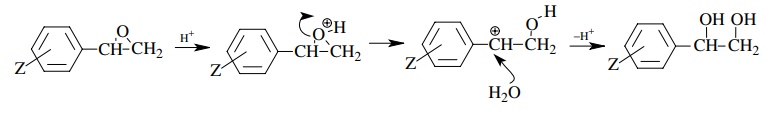
Large
negative ρ+ value consistent
with formation of positive charge on the benzylic carbon. Thus epoxide ring
opening (of protonated epoxide) is the rate-determining step.
