Separation of Azeotropes and Liquids of Similar Volatility
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Evaporation and Distillation
Systems that form azeotropes cannot be separated by fractional distillation, although in some cases, the formation of the azeotrope can be precluded by changing the distillation pressure.
Separation of Azeotropes and
Liquids of Similar Volatility
Systems
that form azeotropes cannot be separated by fractional distillation, although
in some cases, the formation of the azeotrope can be precluded by changing the
distillation pressure. Problems of separation are also found with mixtures of
liquids with similar volatility. Separation of these systems can be facilitated
by adding a third component. If this component forms one or more azeotropes
with the original components of the mixture, the process is called azeotropic
distillation. The addition of a relatively nonvolatile component, which alters
the relative volatility of the original components, gives a process known as
extractive distillation.
In
the azeotropic distillation of minimum-boiling binary mixtures, the third
component forms either a new binary azeotrope of lower boiling point or a
ternary azeotrope of lower boiling point containing the original components in
different proportions. The newly formed azeotrope must be easily separated
after distillation. The process is illustrated by the dehydration of alcohol
with benzene. The binary azeotrope of ethyl alcohol and water boils at 351.15
K, the ternary azeotrope of benzene, water, and alcohol boils at 337.8 K, and
the binary azeotrope of benzene and alcohol boils at 341 K. Distillation of the
alcohol-water azeotrope with benzene yields the ternary azeotrope that
separates on con-densation to give two layers, one of which contains almost all
the water. In a batch process, the column would then give the benzene alcohol
azeotrope, leaving anhydrous alcohol in the still. In a continuous process, the
various stages would each be performed on a different column.
Extractive
distillation is illustrated by the separation of benzene and cyclohexane by
adding phenol. The relative volatility of the original components is modified
so that cyclohexane is recovered as the distillate, leaving a mixture of phenol
and benzene, which is passed to a second column for separation. The phenol,
which is added to the top of the column, appears to aid separation by
preferentially dissolving benzene during its passage downward. This leads to
the term extractive distillation.
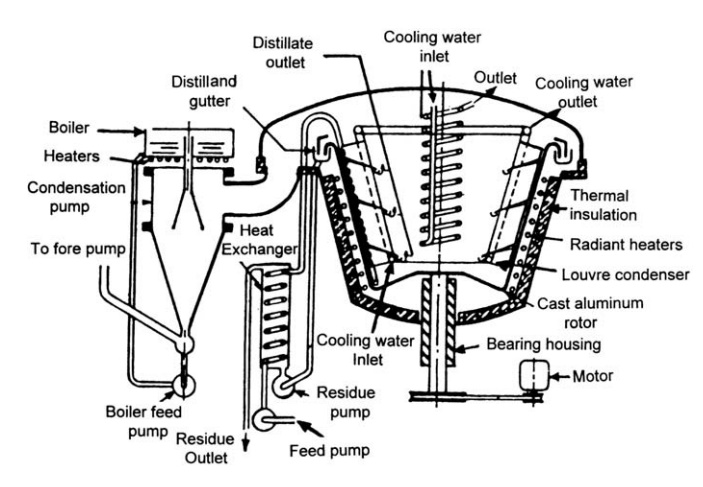
FIGURE 10.8 Large-scale molecular still.
Related Topics
