SAR of Benzodiazepines
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Sedatives and Hypnotics
The presence of electron attracting substituents (Cl, F, Br, NO2) at position C-7 is required for the activity, and the more electron attracting substituents leads to potent activity.
SAR of Benzodiazepines • The presence of electron attracting substituents (Cl, F, Br, NO2) at position C-7 is required for the activity, and the more electron attracting substituents leads to potent activity. • Position 6, 8, and 9 should be unsubstituted for the activity. • Phenyl (or) pyridyl at the C-5 position promotes activity. If the phenyl ring substituted with electron attracting groups at 2’ or 2’, 6’ position, then the activity is increased. • On the other hand, substituents at 3’, 4’, and 5’ positions decreases activity greatly. • Saturation of 4, 5 double bond or shift of it to the 3, 4 position decreases the activity. • Alkyl substitution at position 3 decreases the activity, but the presence or absence of hydroxyl group is essential. Compounds without 3-hydroxyl group are nonpolar and usually have long half-life. Compounds with the 3-hydroxyl group have short half-life because of rapid conjugation with glucuronic acid. • Substitution at N1 by alkyl, halo alkyl, and amino alkyl group increases the activity. • Reduction of carbonyl function at C-2 position to CH2 yields less potent compound. • Triazolo benzodiazepine (Alprazolam) is found to be more potent, they do not require any substitution at 7th position.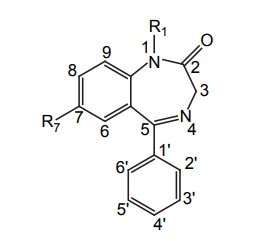
Related Topics
