Review questions answers
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Dosage forms - Emulsions
Pharmaceutical Drugs and Dosage: Dosage forms - Emulsions - Review questions answers
Review questions
17.1 Coalescence
can be reduced by
A. Decreasing the
difference between the density of the dispersed phase and the density of the
medium
B. Adding an agent that
reduces the viscosity of the medium
C. Increasing the
droplet size of the dispersed phase
D. All of the above
17.2 When
compounding an emulsion that contains a flavoring agent, the flavoring agent should
be in the
A. Continuous phase
B. Discontinuous phase
C. Aqueous phase
D. Oil phase
E. Emulsifier
17.3 Define
and differentiate between the following:
A. Creaming and
breaking
B. Creaming and
sedimentation
C. Coalescence and
aggregation
D. Phase inversion and
self-emulsification
E. Multiple emulsions
and microemulsions
F. SEDDS and SMEDDS
17.4 Explain
how sedimentation and creaming in emulsions can be minimized. 17.5 Why is a surfactant
needed to make stable emulsions? Explain which properties of a surfactant are important in formulating emulsions.
Enlist two factors that determine whether an emulsion is o/w or w/o.
17.6 List the
three mechanisms of emulsification.
17.7 What are
emulsifying agents? List the three types of emulsifying agents and differences in their
mechanism of stabilization of an emulsion, for example, in terms of the type of
film formed around the dispersed phase and the zeta potential on the dispersed
phase.
17.8 Which
surfactants will you select for o/w and w/o emulsification.
17.9 Identify
the type of self-emulsifying system most appropriate for the following statements
(SEDDS or SMEDDS):
A. Has lower
dispersed-phase globule size after emulsification
B. Has higher content
of oil
C. Has higher content
of cosolvent
D. Is transparent in
appearance after emulsification
E. Is likely to have
higher oral bioavailability
17.10 Which of
the following surfactants is suitable for the formulation of a o/w emulsion?
A. Surfactant with an
HLB value of 1–3
B. Surfactant with an
HLB value of 3–6
C. Surfactant with an
HLB value of 6–9
D. Surfactant with an
HLB value of 9–12
E. Surfactant with an
HLB value of 12–15
F. Surfactant with an
HLB value of 15–18
17.11 Which of
the following surfactants is suitable for the formulation of a w/o emulsion?
A. Surfactant with an
HLB value of 1–3
B. Surfactant with an
HLB value of 3–6
C. Surfactant with an
HLB value of 6–9
D. Surfactant with an
HLB value of 9–12
E. Surfactant with an
HLB value of 12–15
F. Surfactant with an
HLB value of 15–18
Answers:
17.1 A.
17.2 A.
17.3 A. Creaming
and breaking: Creaming is the upward movement of
dispersed droplets relative to the continuous phase and it is a reversible
process. In contrast, breaking is irreversible. When breaking occurs, simple
mixing fails to resuspend the globules in a stable emulsified form, since the
film surrounding the particles has been destroyed and the oil tends to
coalesce.
B. Creaming and
sedimentation: Creaming is the upward movement of dispersed droplets relative to the
continuous phase, whereas sedimentation is
the downward movement of particles.
C. Coalescence and
aggregation: Coalescence is the process by which the emulsified particles merge with
each other to form large par-ticles. Coalescence is an irreversible process
because the film that surrounds the individual globules is destroyed. In
aggregation, dispersed droplets come together but do not fuse. Aggregation is
to some extent reversible.
D. Phase inversion: An emulsion is
said to invert when it changes from
an o/w to a w/o emulsion or vice versa. Phase inversion can occur by the
addition of an electrolyte or by changing the phase:volume ratio. Monovalent
cations tend to form o/w emul-sions, whereas divalent cations tend to form w/o
emulsions. An o/w emulsion stabilized with sodium stearate can be inverted to a
w/o emulsion by adding calcium chloride to form calcium stearate.
17.4 Creaming is the upward movement of dispersed droplets relative to the continuous phase,
whereas sedimentation is the downward movement of particles. Factors that
influence the rate of creaming are similar to those involved in the rate of
sedimentation. According to Stokes’ law,
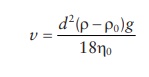
where:
v is the velocity of
creaming
d is the globule
diameter
ρ
and ρ0 are the densities
of dispersed phase and dispersion medium, respectively
η
is the viscosity of the dispersion medium (poise)
g is the acceleration
of gravity (981 cm/s2)
According
to this equation, we can minimize sedimentation and creaming phenomena by
·
A reduction in the globule size
·
A decrease in the density difference between the two phases
·
An increase in the viscosity of the continuous phase
17.5 A.
Increased free energy at the interface occurs because the increase in surface area of
dispersed phase is responsible for the instability of the emulsion. The
surfactants deposit on the interface between the two liquid phases and reduce
the interfacial tension and free energy at the interface.
B. HLB value of surfactant and relative concentration of the
two phases.
17.6 Emulsifying
agents form a film around the dispersed globules to pre-vent coalescence and
thus avoid the separation of two immiscible liq-uids used for emulsion
formation. Emulsifying agents aid in forming emulsions through three different
approaches: (1) reduction of inter-facial tension, (2) formation of a rigid
interfacial film, and (3) forma-tion of an electrical double layer. The film
can act as a mechanical barrier to the coalescence of the globules. An
electrical double layer minimizes coalescence by producing electrical forces
that repulse approaching droplets. Emulsifying agents can be divided into three
groups: (1) surfactants, (2) hydrophilic colloids, and (3) finely divided solid
particles.
·
Surfactants are adsorbed at
oil–water interfaces to form
mono-molecular films and reduce interfacial tensions.
·
Hydrophilic colloids
are
used as emulsifying agents. These include
proteins (gelatin and casein) and polysaccharides (acacia, cellulose
derivatives, and alginates). These materials adsorb at the oil–water interface
and form multilayer films around the
dispersed droplets of oil in an o/w emulsion. Hydrated lyophilic colloids
differ from sur-factants as they do not cause an appreciable lowering in
interfacial tension.
·
Finely divided solid
particles are
adsorbed at the interface between two
immiscible liquid phases and form a film
of par-ticles around the dispersed globules. Finely divided solid
par-ticles are concentrated at the interface, where they produce a particulate
film around the dispersed droplets so as to prevent coalescence.
17.7 In
general, o/w emulsions are formed when the HLB of the surfactants is within the range of
about 9–12, and w/o emulsions are formed when the range is about 3–6.
17.8 A
surfactant with a high HLB value (~9–12) is used as an emulsifier to form o/w emulsions;
and a surfactant of low HLB value (~3–6) to form w/o emulsions.
17.9 A. SEDDS
B. SEDDS
C. SMEDDS
D. SMEDDS
E. SMEDDS
17.10 D.
17.11 B.
