Renal Function and Renal Failure
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Excretion of Drugs
Renal function can be determined by measuring the GFR. Both endogenous and exogenous substances have been used as markers to measure GFR.
RENAL FUNCTION AND RENAL FAILURE
Renal function can be determined by measuring the
GFR. Both endogenous and exogenous substances
have been used as markers to measure GFR. In
order to be useful as a marker, the
agent should entirely get excreted in unchanged form by glomerular filtration
only and should be physiologically and pharmacologically inert. The rate at
which these markers are excreted in
urine reflects the GFR and changes in GFR reflects renal dysfunction. Inulin
(the exogenous fructose polysaccharide) and serum creatinine level have been
used successfully for such purposes.
Inulin clearance provides an accurate measure of
GFR but has the disadvantage of being a
tedious method. Clinically, creatinine clearance is widely used to assess renal
function.
Creatinine is an endogenous amine produced
as a result of muscle catabolism. It is excreted
unchanged in the urine by glomerular filtration only. An advantage of this test
is that it can be correlated to the steady-state concentration of creatinine in
plasma and needs no collection of urine. The method involves determination of
serum creatinine levels. Since creatinine production varies with age, weight
and gender, different formulae are used to calculate creatinine clearance from
the serum creatinine values.
For Children (between 1 to 20 years),
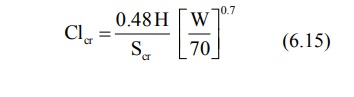
For Adults (above 20 years),
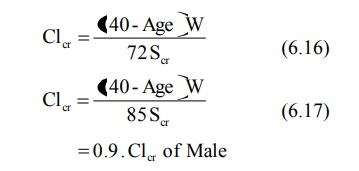
where, Clcr = creatinine clearance in
ml/min,
Scr = serum creatinine in mg%,
H = height in cms, and
W = weight in Kg.
Age is measured in years.
A direct method for determining creatinine
clearance is determination of the amount of creatinine excreted in urine in 24
hours (to calculate the rate of creatinine excretion) and the mean of serum
creatinine from blood samples taken just before and immediately after the urine
collection period. Following formula is used:
ClR = Rate of excretion creatinine / in
creatinine Serum mg % (6.18)
The normal creatinine clearance value is 120 to 130
ml/min. A value of 20 to 50 ml/min denotes moderate renal failure and values
below 10 ml/min indicate severe renal impairment.
The renal function, RF is calculated by equation
6.19.
RF = Clcr of patient / Clcr
of a person normal (6.18)
Dose Adjustment in Renal Failure
Generally speaking, drugs in patients with renal
impairment have altered pharmacokinetic profile. Their renal clearance and
elimination rate are reduced, the elimination half-life is increased and the
apparent volume of distribution is altered. Thus, dose must be altered
depending upon the renal function in such patients. However, except for drugs
having low therapeutic indices, the therapeutic range of others is sufficiently
large and dosage adjustment is not essential.
Dosage regimen need not be changed when
·
The fraction of drug excreted
unchanged, fu is ≤ 0.3, and
·
The renal function RF is ≥ 0.7 of
normal.
The above generalization is based on the assumption
that the metabolites are inactive and binding characteristics and drug
availability are unaltered and so is the renal function in kidney failure conditions.
When the fu value approaches unity and RF approaches zero, elimination
is extremely slowed down and dosing should be reduced drastically. The
significance of nonrenal clearance increases in such conditions.
The required dose in patients with renal impairment
can be calculated by the simple formula:
Drug dose in renal impairment = Normal dose x RF (6.20)
The dosing interval in hours can be computed from
the following equation:
Dosing interval = Normal interval in hours / RF (6.21)
When the drug is eliminated both by renal and
nonrenal mechanisms, the dose to be administered in patients with renal failure
is obtained from equation 6.22.
Drug dose = Normal dose RF x Fraction excreted in
urine + Fraction eliminated nonrenally
(6.22)
Dialysis and Haemoperfusion
In severe renal failure, the patients are put on
dialysis to remove toxic waste products and drugs and their metabolites which
accumulate in the body.
Dialysis is a process in which easily diffusible substances are separated from
poorly diffusible ones by the use of
semipermeable membrane.
There are two procedures for dialysis:
1. Peritoneal dialysis, and
2. Haemodialysis.
In the former, the semipermeable membrane is the
natural membrane of the peritoneal cavity. The method involves introduction of
the dialysate fluid into the abdomen by inserting the catheter and draining and
discarding the same after a certain period of time. In haemodialysis, the
semipermeable membrane is an artificial membrane. Since the system is outside
the body, it is also called as extracorporeal
dialysis. The equipment is referred to as artificial kidney or
haemodialyser. Apart from the removal of toxic waste from the body, haemodialysis is also useful in the
treatment of overdose or poisoning situations where rapid removal of drug
becomes necessary to save the life of the patient. Patients of kidney failure
require dialysis of blood every 2 days. Each treatment period lasts for 3 to 4
hours.
Factors that govern the removal of substances by
haemodialysis are:
Water Solubility: Only water-soluble substances are
dialyzed; lipid soluble drugs such as
glutethimide cannot be removed by dialysis.
Molecular Weight: Molecules with size less than 500
Daltons are dialyzed easily, e.g. many
unbound drugs; drugs having large molecular weight such as vancomycin cannot be
dialyzed.
Protein Binding: Drugs bound to plasma proteins or
blood cells cannot be dialyzed since
dialysis is a passive diffusion process.
Volume of Distribution: Drugs
with large volume of distribution are extensively distributed throughout the body and therefore less easily removed
by dialysis, e.g. digoxin.
The Fig. 6.4 shows schematic representation of
haemodialysis.
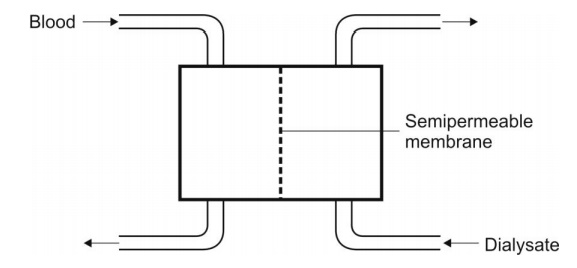
Fig. 6.4. Diagrammatic representation of a haemodialyser. The blood and the
dialysate flow counter-currently.
The dialyzing fluid contains sodium, potassium,
calcium, chloride and acetate ions, and dextrose and other constituents in the
same concentration as that in plasma. The unwanted metabolites in the patient’s
blood such as urea, uric acid, creatinine, etc. diffuse into the dialysate
until equilibrium. Since the volume of dialysate is much greater than that of
blood and since it is replenished with fresh fluid from time to time, almost
complete removal of unwanted substances from the blood is possible. Drugs which
can be removed by haemodialysis are barbiturates, aminoglycosides, chloral
hydrate, lithium, etc.
The rate at which a drug is removed by the dialyser depends upon the flow rate of blood to the machine and its performance. The term dialysance, also called as dialysis clearance, is used to express the ability of machine to clear the drug from blood. It is defined in a manner similar to clearance by equation:
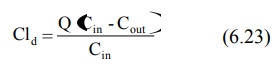
where, Cld = dialysance or dialysis clearance
Q = blood flow rate to dialyser
Cin = concentration of drug in blood
entering the dialyser
Cout = concentration of drug in blood
leaving the dialyser
In haemoperfusion,
the blood is passed through a bed of adsorbent such as charcoal or resin; as a
result, drugs and other unwanted molecules are adsorbed while plasma proteins
are not. The method is also useful in treating severe drug intoxication. The
limitation of haemoperfusion is that it also removes the blood platelets, white
cells and endogenous steroids.
Related Topics
