Relevant Physiology of Urine Formation
| Home | | Pharmacology |Chapter: Essential pharmacology : Drugs Acting On Kidney
Urine formation starts from glomerular filtration (g.f.) in a prodigal way. Normally, about 180 L of fluid is filtered everyday: all soluble constituents of blood minus the plasma proteins (along with substances bound to them) and lipids, are filtered at the glomerulus.
RELEVANT PHYSIOLOGY
OF URINE FORMATION
Urine formation starts
from glomerular filtration (g.f.) in a prodigal way. Normally, about 180 L of
fluid is filtered everyday: all soluble constituents of blood minus the plasma
proteins (along with substances bound to them) and lipids, are filtered at the
glomerulus. More than 99% of the glomerular filtrate is reabsorbed in the
tubules; about 1.5 L urine is produced in 24 hours. The diuretics act primarily
by inhibiting tubular reabsorption: just 1% decrease in tubular reabsorption
would more than double urine output.
The mechanisms that
carryout ion movement across tubular cells are complex and involve a variety of
energy dependent transmembrane pumps as well as channels in between the loose
fitting cells of the proximal tubule (PT). All Na+ that enters tubular cells
through the luminal membrane is pumped out of it into the renal interstitium at
the basolateral membrane by Na+K+ATPase energised Na+K+ antiporter (see Figs 41.1 and 41.2). Because there
is a large intracellular to extracellular gradient for K+, it diffuses out
through K+ channels to be recirculated by the Na+K+ antiporter. For
simplification, tubular reabsorption can be divided into four sites (Fig.
IX.1).
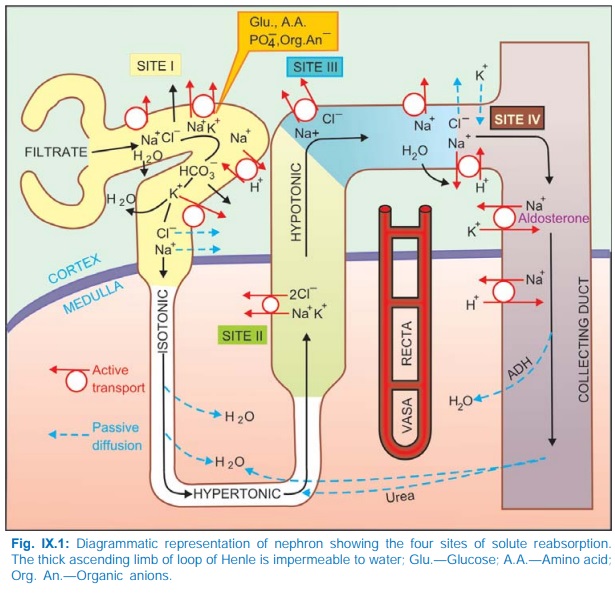
Site I: Proximal Tubule
Four mechanisms of Na+ transport have been
defined in this segment.
a) Direct entry of Na+
along a favourable electrochemical gradient. This is electrogenic.
b) Transport of Na+ and
K+ coupled to active reabsorption of glucose, amino acids, other organic anions
and PO34¯ through specific symporters. Only the glucose coupled Na+
reabsorption is electrogenic.
c) Exchange with H+: The PT cells secrete H+ with the help of carbonic anhydrase (CAse), Fig. IX.2. H+ ion exchanges with Na+ present in tubular fluid through Na+H+ antiporter located in the luminal membrane and forms H2CO3 by combining with HCO¯. This H2 CO3 is broken into H2O + CO2 by brush border CAse; both CO2 and H2O diffuse inside the cell and recombine to form H2CO3 (intracellular CAse catalysed reaction) which is the source of H+. The dissociated HCO3¯ in the cell is transported to cortical e.c.f. by basolateral membrane Na+HCO¯ symporter resulting in net reabsorption of NaHCO3. Practically all HCO3¯ is reabsorbed in PT by this mechanism, because tubular membrane, as such, is relatively impermeable to HCO3¯.
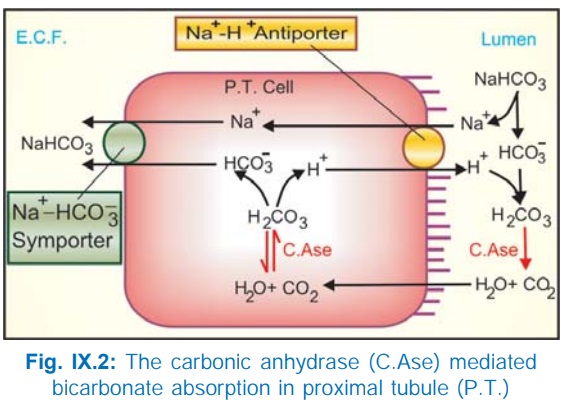
d) The
disproportionately large HCO3¯, acetate, PO43¯,
amino acid and other anion reabsorption create passive driving forces for Cl¯
to diffuse through the paracellular pathway (in between tubular cells),
particularly in the later PT. This takes Na+ and water along to maintain
electrical neutrality and isotonicity; reabsorption in PT is isotonic.
Major part of filtered
K+ is reabsorbed in the PT. Thus, an isotonic tubular fluid with major changes
in composition enters the thin descending limb of loop of Henle.
Site II: Ascending Limb Of Loop Of Henle (Asc LH)
The thick AscLH can be
distinguished into two distinct portions:
·
Medullary part lined by cuboidal cells.
·
Cortical part lined by flattened cells.
Both portions are relatively
impermeable to water but absorb salt actively and thus dilute the tubular
fluid.
In the medullary portion
a distinct luminal membrane carrier transports ions in the stoichiometric ratio
of Na+K+2 Cl¯ (see Fig. 41.1), and is
non-electrogenic. The Na+ that enters the cell is pumped to e.c.f. by Na+ K+
ATPase at the basolateral membrane. In addition, a Na+Cl¯ symporter moves Cl¯ down
its electrochemical gradient into e.c.f. and carries Na+ along. As the tubular
fluid traverses AscLH it progressively becomes hypotonic. Accumulation of NaCl
in the medullary interstitium without accompanying water makes it hypertonic: a
corticomedullary osmotic gradient is set up. This draws in water from the
descending limb of loop of Henle (this thin segment has high osmotic water
permeability but lacks active NaCl transport) so that the fluid that enters
AscLH becomes hypertonic. A 4 times higher osmolarity of medullary tip
(papilla) is maintained by the hairpin structure of the loop of Henle acting as
passive counter current multiplier
and the arrangement of blood vessels as vasa
recti with shunts that prevents washing away of the osmotic gradient by
progressively reducing blood flow to the inner medulla. Because of meagre blood
supply, renal papilla is so prone to necrosis and suffers maximum damage when a
toxic substance is being excreted.
Site III: Cortical Diluting Segment Of Loop Of Henle
This segment, also
impermeable to water, continues to
absorb salt, but here it is through a Na+Cl¯ symporter. Tubular fluid gets
further diluted.
Site IV: Distal Tubule (DT) And Collecting Duct (CD)
In the late DT and CD,
Na+ is again actively reabsorbed; the cation-anion balance
being maintained partly by passive Cl¯ diffusion and partly by secretion of K+
and H+. Absorption of Na+ at this site occurs through a specific amiloride
sensitive Na+ channel and is controlled to a large extent by aldosterone (see
Fig. 41.3). This provides fine tuning to electrolyte excretion according to
body needs.
In common with other
cells, the DT and CD cells are rich in K+; a chemical gradient exists for its
diffusion into tubular lumen which is aided by the lumen negative transepithelial
potential difference in this part of the tubule. The luminal membrane possesses
an active secretory pump for H+ which is again governed by movement of Na+ in
the reverse direction. Any diuretic acting proximal to the aldosterone sensitive
ion exchange site causes an increased delivery of Na+ to the distal
nephron—more exchange with K+ takes place. Thus, K+ is reabsorbed in the PT and
AscLH, and is secreted in the DT and CD. The net K+ loss is regulated by
variations in the secretory process and depends on:
·The Na+
load delivered to distal segment
·Presence or absence of
aldosterone
·Availability of H+
·Intracellular K+
stores
The characteristic
feature of cells lining CD is their responsiveness to antidiuretic hormone
(ADH). If ADH is absent, the hypotonic fluid entering CD is passed as such → dilute urine is
produced during water loading. If ADH levels are high, CD cells become fully permeable
to water → equilibrate with
hyperosmotic medulla → concentrated urine is passed, as occurs during
water deprivation or hypertonic saline infusion.
The CD and thin AscLH
are the only segments permeable to urea. ADH promotes insertion of urea transporter
(UT1 or VRUT) into the luminal membrane of CD cells → more urea is
accumulated in the medullary interstitium, reinforcing the medullary
hypertonicity during water deprivation.
Free Water Clearance
It is defined as the volume of urine excreted per unit time in
excess of that required to excrete the contained solute iso-osmotically with
plasma. It is positive when dilute urine is passed in the absence of ADH and
negative when concentrated urine is passed in the presence of ADH. If isotonic
urine is passed, regardless of its volume, free water clearance is zero.
Both positive and
negative free water clearance are dependent on the production of a corticomedullary
osmotic gradient; diuretics acting on medullary AscLH depress both.
Organic Ion Transport
The PT has non-specific
bidirectional active transport mechanism, separately for organic acids and
organic bases. However, the magnitude of transport in the two directions may
vary from compound to compound, e.g. reabsorption of uric acid is generally
more than its secretion, while in case of penicillin the converse is true.
Important diuretics like furosemide, thiazides and amiloride utilize this
transport to approach their site of action from the luminal side of the tubule
in the AscLH/DT/CD.
Regulation Of Renal
Function
Glomerular filtration
rate (g.f.r.) is dependent on the pumping action of heart, the magnitude of
renal blood flow and the relative dimensions of afferent and efferent
glomerular vessels. Thus, systemic and intrarenal haemodynamic changes can
reflect in g.f.r.
About 80% nephrons lie
in outer cortex, have short loops of Henle and low Na+ reabsorptive capacity;
while 20% or so are juxtamedullary, possess long loops of Henle and are largely
responsible for creating the corticomedullary osmotic gradient. Redistribution
of blood flow between these two types of nephrons can alter salt and water
excretion. Further, haemodynamic changes within different segments of renal
vasculature can alter pressure relationships which govern flow of solute and
water.
The renin-angiotensin aldosterone
system has a profound bearing on distal tubular reabsorption of Na+ and
secretion of K+/H+. Angiotensin II produced locally in the kidney has direct
effects on intrarenal vascular beds as well as on salt and water reabsorption.
Sympathetic stimulation of kidney results in renin release which
would indirectly affect tubular transport. In addition, adrenergic drugs can
directly enhance reabsorption of salt and water.
Prostaglandins (PGs) are produced locally in kidney; act as
modulators of renal circulation and renin release. PGE2 inhibits the
action of ADH and has direct effects on tubular reabsorption.
A natriuretic hormone produced by the atrium (atrial natriuretic
peptide: ANP) and may be other sites also has been found to be important in
inducing natriuresis in response to salt and volume overload. It mediates
‘escape’ from long-term aldosterone action.
All nephrons are so arranged that the Asc LH passes close to the
early PT of the same nephron. The macula densa cells are thus in close contact
with afferent and efferent arterioles. This provides opportunity for feedback regulation
of single unit function.
Relation To Diuretic Action
The relative
magnitudes of Na+ reabsorption at different tubular sites are:
PT 65–70%; Asc LH 20–25%;
DT 8–9%; CD 1–2%.
The maximal
natriuretic response to a diuretic can give a clue to its site of action. It
may appear that diuretics acting on PT should be the most efficacious. However,
these agents are either too weak or cause distortion of acidbase balance (CAse
inhibitors). Further, their effect may be obscured by compensatory increase in
reabsorption further down the nephron, because the reserve reabsorptive
capacity of diluting segments is considerable and can overshadow more proximal
actions.
A diuretic having
primary action on medullary Asc LH (furosemide) can produce substantial effect
because of limited capacity for salt absorption in DT and CD. This also
explains why agents acting on DT and CD (K+ sparing diuretics) evoke only mild
saluretic effect. Diuretics acting on cortical diluting segment (thiazides) are
intermediate between these two.
Related Topics
